Diatom mimics: directing the formation of biosilica nanoparticles by controlled folding of lysine-leucine peptides
- PMID: 25285787
- PMCID: PMC4608251
- DOI: 10.1021/ja5078238
Diatom mimics: directing the formation of biosilica nanoparticles by controlled folding of lysine-leucine peptides
Abstract
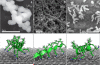
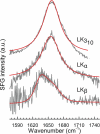
Silaffins, long chain polyamines, and other biomolecules found in diatoms are involved in the assembly of a large number of silica nanostructures under mild, ambient conditions. Nanofabrication researchers have sought to mimic the diatom's biosilica production capabilities by engineering proteins to resemble aspects of naturally occurring biomolecules. Such mimics can produce monodisperse biosilica nanospheres, but in vitro production of the variety of intricate biosilica nanostructures that compose the diatom frustule is not yet possible. In this study we demonstrate how LK peptides, composed solely of lysine (K) and leucine (L) amino acids arranged with varying hydrophobic periodicities, initiate the formation of different biosilica nanostructures in vitro. When L and K residues are arranged with a periodicity of 3.5 the α-helical form of the LK peptide produces monodisperse biosilica nanospheres. However, when the LK periodicity is changed to 3.0, corresponding to a 310 helix, the morphology of the nanoparticles changes to elongated rod-like structures. β-strand LK peptides with a periodicity of 2.0 induce wire-like silica morphologies. This study illustrates how the morphology of biosilica can be changed simply by varying the periodicity of polar and nonpolar amino acids.
Figures


Similar articles
-
Silaffins as functional biomacromolecules in regulating frustule morphogenesis and biosilica properties.Int J Biol Macromol. 2025 May;309(Pt 4):143105. doi: 10.1016/j.ijbiomac.2025.143105. Epub 2025 Apr 11. Int J Biol Macromol. 2025. PMID: 40222518
-
Reconstituting the formation of hierarchically porous silica patterns using diatom biomolecules.J Struct Biol. 2018 Oct;204(1):64-74. doi: 10.1016/j.jsb.2018.07.005. Epub 2018 Jul 29. J Struct Biol. 2018. PMID: 30009877
-
Polycationic peptides from diatom biosilica that direct silica nanosphere formation.Science. 1999 Nov 5;286(5442):1129-32. doi: 10.1126/science.286.5442.1129. Science. 1999. PMID: 10550045
-
Preparation of biosilica structures from frustules of diatoms and their applications: current state and perspectives.Appl Microbiol Biotechnol. 2013 Jan;97(2):453-60. doi: 10.1007/s00253-012-4568-0. Epub 2012 Nov 18. Appl Microbiol Biotechnol. 2013. PMID: 23179621 Review.
-
Harnessing Microalgae as Sustainable Cell Factories for Polyamine-Based Nanosilica for Biomedical Applications.Molecules. 2025 Apr 8;30(8):1666. doi: 10.3390/molecules30081666. Molecules. 2025. PMID: 40333571 Free PMC article. Review.
Cited by
-
Bioinspired Scaffolding by Supramolecular Amines Allows the Formation of One- and Two-Dimensional Silica Superstructures.Chemistry. 2020 Nov 26;26(66):15330-15336. doi: 10.1002/chem.202003139. Epub 2020 Oct 19. Chemistry. 2020. PMID: 32783243 Free PMC article.
-
Intrinsically Disordered Osteopontin Fragment Orders During Interfacial Calcium Oxalate Mineralization.Angew Chem Int Ed Engl. 2021 Aug 16;60(34):18577-18581. doi: 10.1002/anie.202105768. Epub 2021 Jul 16. Angew Chem Int Ed Engl. 2021. PMID: 34118104 Free PMC article.
-
Peptide-equipped tobacco mosaic virus templates for selective and controllable biomineral deposition.Beilstein J Nanotechnol. 2015 Jun 25;6:1399-412. doi: 10.3762/bjnano.6.145. eCollection 2015. Beilstein J Nanotechnol. 2015. PMID: 26199844 Free PMC article.
-
Tipping the Scale from Disorder to Alpha-helix: Folding of Amphiphilic Peptides in the Presence of Macroscopic and Molecular Interfaces.PLoS Comput Biol. 2015 Aug 21;11(8):e1004328. doi: 10.1371/journal.pcbi.1004328. eCollection 2015 Aug. PLoS Comput Biol. 2015. PMID: 26295346 Free PMC article.
-
Nanoengineered Silica-Based Biomaterials for Regenerative Medicine.Int J Mol Sci. 2024 Jun 1;25(11):6125. doi: 10.3390/ijms25116125. Int J Mol Sci. 2024. PMID: 38892312 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

