Short hairpin RNA library-based functional screening identified ribosomal protein L31 that modulates prostate cancer cell growth via p53 pathway
- PMID: 25285958
- PMCID: PMC4186824
- DOI: 10.1371/journal.pone.0108743
Short hairpin RNA library-based functional screening identified ribosomal protein L31 that modulates prostate cancer cell growth via p53 pathway
Abstract
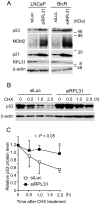
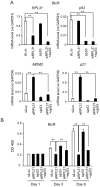
Androgen receptor is a primary transcription factor involved in the proliferation of prostate cancer cells. Thus, hormone therapy using antiandrogens, such as bicalutamide, is a first-line treatment for the disease. Although hormone therapy initially reduces the tumor burden, many patients eventually relapse, developing tumors with acquired endocrine resistance. Elucidation of the molecular mechanisms underlying endocrine resistance is therefore a fundamental issue for the understanding and development of alternative therapeutics for advanced prostate cancer. In the present study, we performed short hairpin RNA (shRNA)-mediated functional screening to identify genes involved in bicalutamide-mediated effects on LNCaP prostate cancer cells. Among such candidate genes selected by screening using volcano plot analysis, ribosomal protein L31 (RPL31) was found to be essential for cell proliferation and cell-cycle progression in bicalutamide-resistant LNCaP (BicR) cells, based on small interfering RNA (siRNA)-mediated knockdown experiments. Of note, RPL31 mRNA is more abundantly expressed in BicR cells than in parental LNCaP cells, and clinical data from ONCOMINE and The Cancer Genome Altas showed that RPL31 is overexpressed in prostate carcinomas compared with benign prostate tissues. Intriguingly, protein levels of the tumor suppressor p53 and its targets, p21 and MDM2, were increased in LNCaP and BicR cells treated with RPL31 siRNA. We observed decreased degradation of p53 protein after RPL31 knockdown. Moreover, the suppression of growth and cell cycle upon RPL31 knockdown was partially recovered with p53 siRNA treatment. These results suggest that RPL31 is involved in bicalutamide-resistant growth of prostate cancer cells. The shRNA-mediated functional screen in this study provides new insight into the molecular mechanisms and therapeutic targets of advanced prostate cancer.
Conflict of interest statement
Figures


Similar articles
-
Interleukin-6 increases prostate cancer cells resistance to bicalutamide via TIF2.Mol Cancer Ther. 2009 Mar;8(3):665-71. doi: 10.1158/1535-7163.MCT-08-0823. Epub 2009 Feb 24. Mol Cancer Ther. 2009. PMID: 19240160 Free PMC article.
-
Niclosamide and Bicalutamide Combination Treatment Overcomes Enzalutamide- and Bicalutamide-Resistant Prostate Cancer.Mol Cancer Ther. 2017 Aug;16(8):1521-1530. doi: 10.1158/1535-7163.MCT-16-0912. Epub 2017 May 12. Mol Cancer Ther. 2017. PMID: 28500234 Free PMC article.
-
Modulators of prostate cancer cell proliferation and viability identified by short-hairpin RNA library screening.PLoS One. 2012;7(4):e34414. doi: 10.1371/journal.pone.0034414. Epub 2012 Apr 11. PLoS One. 2012. PMID: 22509301 Free PMC article.
-
Targeting prostate cancer based on signal transduction and cell cycle pathways.Cell Cycle. 2008 Jun 15;7(12):1745-62. doi: 10.4161/cc.7.12.6166. Epub 2008 Jun 16. Cell Cycle. 2008. PMID: 18594202 Free PMC article. Review.
-
Translational and clinical implications of the genetic landscape of prostate cancer.Nat Rev Clin Oncol. 2016 Oct;13(10):597-610. doi: 10.1038/nrclinonc.2016.76. Epub 2016 Jun 1. Nat Rev Clin Oncol. 2016. PMID: 27245282 Free PMC article. Review.
Cited by
-
Dynamics of Colon Monocyte and Macrophage Activation During Colitis.Front Immunol. 2018 Nov 27;9:2764. doi: 10.3389/fimmu.2018.02764. eCollection 2018. Front Immunol. 2018. PMID: 30542349 Free PMC article.
-
Silencing of HMGA2 promotes apoptosis and inhibits migration and invasion of prostate cancer cells.J Biosci. 2016 Jun;41(2):229-36. doi: 10.1007/s12038-016-9603-3. J Biosci. 2016. PMID: 27240983
-
Replication-dependent histone isoforms: a new source of complexity in chromatin structure and function.Nucleic Acids Res. 2018 Sep 28;46(17):8665-8678. doi: 10.1093/nar/gky768. Nucleic Acids Res. 2018. PMID: 30165676 Free PMC article. Review.
-
Comprehensive analysis of somatic copy number alterations in clear cell renal cell carcinoma.Mol Carcinog. 2020 Apr;59(4):412-424. doi: 10.1002/mc.23164. Epub 2020 Feb 10. Mol Carcinog. 2020. PMID: 32039517 Free PMC article.
-
Ribosomal proteins and human diseases: molecular mechanisms and targeted therapy.Signal Transduct Target Ther. 2021 Aug 30;6(1):323. doi: 10.1038/s41392-021-00728-8. Signal Transduct Target Ther. 2021. PMID: 34462428 Free PMC article. Review.
References
-
- Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, et al. (2007) Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res 67: 5033–5041. - PubMed
-
- Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, et al. (1997) Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res 57: 314–319. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

