A Bayesian meta-analysis of multiple treatment comparisons of systemic regimens for advanced pancreatic cancer
- PMID: 25286060
- PMCID: PMC4186762
- DOI: 10.1371/journal.pone.0108749
A Bayesian meta-analysis of multiple treatment comparisons of systemic regimens for advanced pancreatic cancer
Abstract
Background: For advanced pancreatic cancer, many regimens have been compared with gemcitabine (G) as the standard arm in randomized controlled trials. Few regimens have been directly compared with each other in randomized controlled trials and the relative efficacy and safety among them remains unclear.
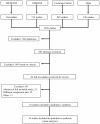
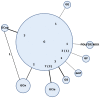
Methods: A systematic review was performed through MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, and ASCO meeting abstracts up to May 2013 to identify randomized controlled trials that included advanced pancreatic cancer comparing the following regimens: G, G+5-fluorouracil, G+ capecitabine, G+S1, G+ cisplatin, G+ oxaliplatin, G+ erlotinib, G+ nab-paclitaxel, and FOLFIRINOX. Overall survival and progression-free survival with 95% credible regions were extracted using the Parmar method. A Bayesian multiple treatment comparisons was performed to compare all regimens simultaneously.
Results: Twenty-two studies were identified and 16 were included in the meta-analysis. Median overall survival, progression free survival, and response rates for G arms from all trials were similar, suggesting no significant clinical heterogeneity. For overall survival, the mixed treatment comparisons found that the probability that FOLFIRINOX was the best regimen was 83%, while it was 11% for G+ nab-paclitaxel and 3% for G+ S1 and G+ erlotinib, respectively. The overall survival hazard ratio for FOLFIRINOX versus G+ nab-paclitaxel was 0.79 [0.50-1.24], with no obvious difference in toxicities. The hazard ratios from direct pairwise comparisons were consistent with the mixed treatment comparisons results.
Conclusions: FOLFIRINOX appeared to be the best regimen for advanced pancreatic cancer probabilistically, with a trend towards improvement in survival when compared with other regimens by indirect comparisons.
Conflict of interest statement
Figures



References
-
- American Cancer Society (2013) Cancer facts and figures 2013. Available: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/docume... Accessed 2013 July 30.
-
- Office for National Statistics (2011) Cancer Survival in England patients diagnosed 2005–2009 followed up to 2010. Available: http://www.ons.gov.uk/ons/rel/cancer-unit/cancer-survival/2005-2009-foll... Accessed 2014 July 4.
-
- Warsame R, Grothey A (2012) Treatment options for advanced pancreatic cancer: A review. Expert Rev Anticancer Ther 12: 1327–36. - PubMed
-
- Li D, Xie K, Wolff R, Abbruzzese JL (2004) Pancreatic cancer. Lancet 363: 1049–57. - PubMed
-
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, et al. (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15: 2403–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

