Neuroprotection of a novel cyclopeptide C*HSDGIC* from the cyclization of PACAP (1-5) in cellular and rodent models of retinal ganglion cell apoptosis
- PMID: 25286089
- PMCID: PMC4186886
- DOI: 10.1371/journal.pone.0108090
Neuroprotection of a novel cyclopeptide C*HSDGIC* from the cyclization of PACAP (1-5) in cellular and rodent models of retinal ganglion cell apoptosis
Abstract
Purpose: To investigate the protective effects of a novel cyclopeptide C*HSDGIC* (CHC) from the cyclization of Pituitary adenylate cyclase-activating polypeptide (PACAP) (1-5) in cellular and rodent models of retinal ganglion cell apoptosis.
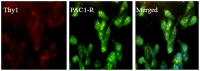
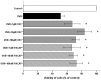
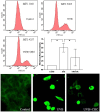
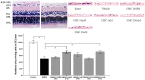
Methodology/principal findings: Double-labeling immunohistochemistry was used to detect the expression of Thy-1 and PACAP receptor type 1 in a retinal ganglion cell line RGC-5. The apoptosis of RGC-5 cells was induced by 0.02 J/cm(2) Ultraviolet B irradiation. MTT assay, flow cytometry, fluorescence microscopy were used to investigate the viability, the level of reactive oxygen species (ROS) and apoptosis of RGC-5 cells respectively. CHC attenuated apoptotic cell death induced by Ultraviolet B irradiation and inhibited the excessive generation of ROS. Moreover, CHC treatment resulted in decreased expression of Bax and concomitant increase of Bcl-2, as was revealed by western-blot analysis. The in vivo apoptosis of retinal ganglion cells was induced by injecting 50 mM N-methyl-D-aspartate (NMDA) (100 nmol in a 2 µL saline solution) intravitreally, and different dosages of CHC were administered. At day 7, rats in CHC+ NMDA-treated groups showed obvious aversion to light when compared to NMDA rats. Electroretinogram recordings revealed a marked decrease in the amplitudes of a-wave, b-wave, and photopic negative response due to NMDA damage. In retina receiving intravitreal NMDA and CHC co-treatment, these values were significantly increased. CHC treatment also resulted in less NMDA-induced cell loss and a decrease in the proportion of dUTP end-labeling-positive cells in ganglion cell line.
Conclusions: C*HSDGIC*, a novel cyclopeptide from PACAP (1-5) attenuates apoptosis in RGC-5 cells and inhibits NMDA-induced retinal neuronal death. The beneficial effects may occur via the mitochondria pathway. PACAP derivatives like CHC may serve as a promising candidate for neuroprotection in glaucoma.
Conflict of interest statement
Figures





References
-
- Nucci C, Strouthidis NG, Khaw PT (2013) Neuroprotection and other novel therapies for glaucoma. Curr Opin Pharmacol 13: 1–4. - PubMed
-
- Vaudry D, Gonzales BJ, Basille M, Yon L, Fournier A, et al. (2000) Pituitary adenylate cyclase activating polypeptide and its receptors: from structure to function. Pharmacol Rev 52: 269–324. - PubMed
-
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, et al. (2009) Pituitary adenylate cyclase activating polypeptide and its receptors: 20 years after thediscovery. Pharmacol Rev 61: 283–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

