Intravenous angiotensin II for the treatment of high-output shock (ATHOS trial): a pilot study
- PMID: 25286986
- PMCID: PMC4212099
- DOI: 10.1186/s13054-014-0534-9
Intravenous angiotensin II for the treatment of high-output shock (ATHOS trial): a pilot study
Abstract
Introduction: Patients with distributive shock who require high dose vasopressors have a high mortality. Angiotensin II (ATII) may prove useful in patients who remain hypotensive despite catecholamine and vasopressin therapy. The appropriate dose of parenteral angiotensin II for shock is unknown.
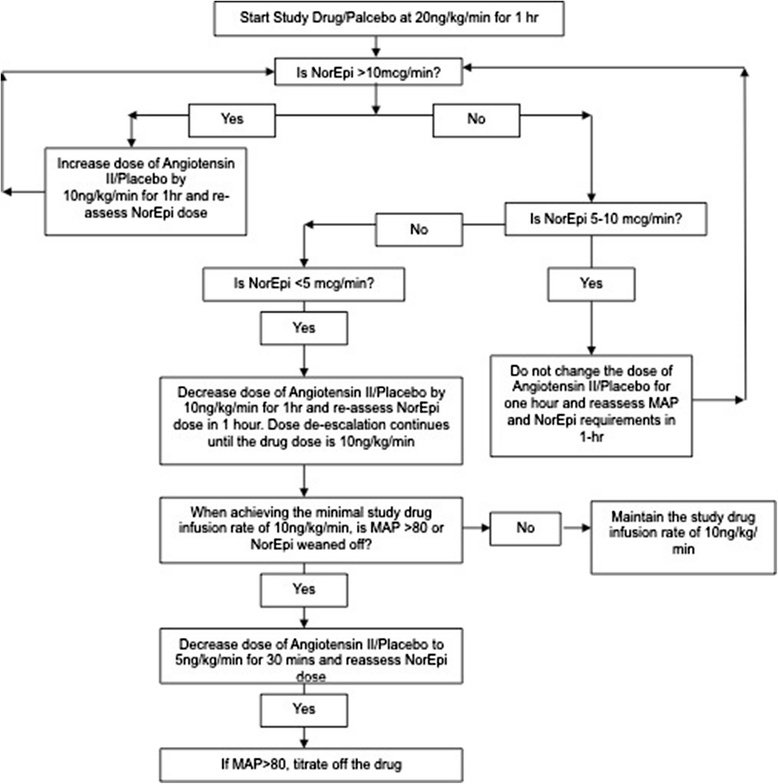
Methods: In total, 20 patients with distributive shock and a cardiovascular Sequential Organ Failure Assessment score of 4 were randomized to either ATII infusion (N =10) or placebo (N =10) plus standard of care. ATII was started at a dose of 20 ng/kg/min, and titrated for a goal of maintaining a mean arterial pressure (MAP) of 65 mmHg. The infusion (either ATII or placebo) was continued for 6 hours then titrated off. The primary endpoint was the effect of ATII on the standing dose of norepinephrine required to maintain a MAP of 65 mmHg.
Results: ATII resulted in marked reduction in norepinephrine dosing in all patients. The mean hour 1 norepinephrine dose for the placebo cohort was 27.6 ± 29.3 mcg/min versus 7.4 ± 12.4 mcg/min for the ATII cohort (P =0.06). The most common adverse event attributable to ATII was hypertension, which occurred in 20% of patients receiving ATII. 30-day mortality for the ATII cohort and the placebo cohort was similar (50% versus 60%, P =1.00).
Conclusion: Angiotensin II is an effective rescue vasopressor agent in patients with distributive shock requiring multiple vasopressors. The initial dose range of ATII that appears to be appropriate for patients with distributive shock is 2 to 10 ng/kg/min.
Trial registration: Clinicaltrials.gov NCT01393782. Registered 12 July 2011.
Figures
Comment in
-
Angiotensin II: a new approach for refractory shock management?Crit Care. 2014 Dec 18;18(6):694. doi: 10.1186/s13054-014-0694-7. Crit Care. 2014. PMID: 25645552 Free PMC article.
References
-
- Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, Orecchioni A, D'Egidio A, D'Ippoliti F, Raffone C, Venditti M, Guarracino F, Girardis M, Tritapepe L, Pietropaoli P, Mebazaa A, Singer M. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310:1683–1691. doi: 10.1001/jama.2013.278477. - DOI - PubMed
-
- Russell JA, Walley KR, Singer J, Gordon AC, Hebert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM, Cook DJ, Presneill JJ, Ayers D, VASST Investigators Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877–887. doi: 10.1056/NEJMoa067373. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical