Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin-2 and TNF-α pathways
- PMID: 25287778
- PMCID: PMC4282702
- DOI: 10.1007/s00262-014-1610-3
Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin-2 and TNF-α pathways
Abstract
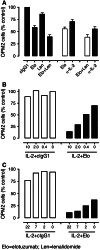
Elotuzumab is a humanized monoclonal antibody specific for signaling lymphocytic activation molecule-F7 (SLAMF7, also known as CS1, CD319, or CRACC) that enhances natural killer (NK) cell-mediated antibody-dependent cellular cytotoxicity (ADCC) of SLAMF7-expressing myeloma cells. This study explored the mechanisms underlying enhanced myeloma cell killing with elotuzumab as a single agent and in combination with lenalidomide, to support ongoing phase III trials in patients with relapsed/refractory or newly-diagnosed multiple myeloma (MM). An in vitro peripheral blood lymphocyte (PBL)/myeloma cell co-culture model was developed to evaluate the combination of elotuzumab and lenalidomide. Expression of activation markers and adhesion receptors was evaluated by flow cytometry, cytokine expression by Luminex and ELISPOT assays, and cytotoxicity by myeloma cell counts. Elotuzumab activated NK cells and promoted myeloma cell death in PBL/myeloma cell co-cultures. The combination of elotuzumab plus lenalidomide demonstrated superior anti-myeloma activity on established MM xenografts in vivo and in PBL/myeloma cell co-cultures in vitro than either agent alone. The combination enhanced myeloma cell killing by modulating NK cell function that coincided with the upregulation of adhesion and activation markers, including interleukin (IL)-2Rα expression, IL-2 production by CD3(+)CD56(+) lymphocytes, and tumor necrosis factor (TNF)-α production. In co-culture assays, TNF-α directly increased NK cell activation and myeloma cell death with elotuzumab or elotuzumab plus lenalidomide, and neutralizing TNF-α decreased NK cell activation and myeloma cell death with elotuzumab. These results demonstrate that elotuzumab activates NK cells and induces myeloma cell death via NK cell-mediated ADCC, which is further enhanced when combined with lenalidomide.
Conflict of interest statement
The investigational drug elotuzumab is being developed in a partnership between AbbVie Biotherapeutics Inc. and Bristol-Myers Squibb Co. Michael Robbins and Audie Rice are currently employees of Bristol-Myers Squibb. All other authors are current or former employees of AbbVie Biotherapeutics Inc.
Figures



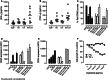

References
-
- Wang M, Dimopoulos MA, Chen C, Cibeira MT, Attal M, Spencer A, Rajkumar SV, Yu Z, Olesnyckyj M, Zeldis JB, Knight RD, Weber DM. Lenalidomide plus dexamethasone is more effective than dexamethasone alone in patients with relapsed or refractory multiple myeloma regardless of prior thalidomide exposure. Blood. 2008;112:4445–4451. doi: 10.1182/blood-2008-02-141614. - DOI - PubMed
-
- Gandhi AK, Kang J, Capone L, Parton A, Wu L, Zhang LH, Mendy D, Lopez-Girona A, Tran T, Sapinosa L, Fang W, Xu S, Hampton G, Bartlett JB, Schafer P. Dexamethasone synergizes with lenalidomide to inhibit multiple myeloma tumor growth, but reduces lenalidomide-induced immunomodulation of T and NK cell function. Curr Cancer Drug Targets. 2010;10:155–167. doi: 10.2174/156800910791054239. - DOI - PubMed
-
- Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, Shirley MA, Muller G, Schafer P, Stirling D, Dalgleish AG, Bartlett JB. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69:56–63. doi: 10.1016/j.mvr.2005.01.002. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

