Regulation and function of adult neurogenesis: from genes to cognition
- PMID: 25287858
- PMCID: PMC4280160
- DOI: 10.1152/physrev.00004.2014
Regulation and function of adult neurogenesis: from genes to cognition
Abstract
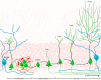
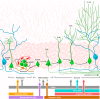
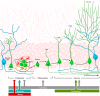
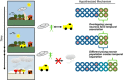
Adult neurogenesis in the hippocampus is a notable process due not only to its uniqueness and potential impact on cognition but also to its localized vertical integration of different scales of neuroscience, ranging from molecular and cellular biology to behavior. This review summarizes the recent research regarding the process of adult neurogenesis from these different perspectives, with particular emphasis on the differentiation and development of new neurons, the regulation of the process by extrinsic and intrinsic factors, and their ultimate function in the hippocampus circuit. Arising from a local neural stem cell population, new neurons progress through several stages of maturation, ultimately integrating into the adult dentate gyrus network. The increased appreciation of the full neurogenesis process, from genes and cells to behavior and cognition, makes neurogenesis both a unique case study for how scales in neuroscience can link together and suggests neurogenesis as a potential target for therapeutic intervention for a number of disorders.
Copyright © 2014 the American Physiological Society.
Figures



References
-
- Aimone JB, Deng W, Gage FH. Put them out to pasture? What are old granule cells good for, anyway? Hippocampus 20: 1124–1125, 2010. - PubMed
-
- Aimone JB, Gage FH. Modeling new neuron function: a history of using computational neuroscience to study adult neurogenesis. Eur J Neurosci 33: 1160–1169, 2011. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

