The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes
- PMID: 25287911
- PMCID: PMC4282969
- DOI: 10.1007/s00401-014-1347-2
The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes
Abstract
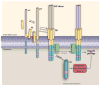
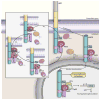
The amyloid precursor protein (APP) has occupied a central position in Alzheimer's disease (AD) pathophysiology, in large part due to the seminal role of amyloid-β peptide (Aβ), a proteolytic fragment derived from APP. Although the contribution of Aβ to AD pathogenesis is accepted by many in the research community, recent studies have unveiled a more complicated picture of APP's involvement in neurodegeneration in that other APP-derived fragments have been shown to exert pathological influences on neuronal function. However, not all APP-derived peptides are neurotoxic, and some even harbor neuroprotective effects. In this review, we will explore this complex picture by first discussing the pleiotropic effects of the major APP-derived peptides cleaved by multiple proteases, including soluble APP peptides (sAPPα, sAPPβ), various C- and N-terminal fragments, p3, and APP intracellular domain fragments. In addition, we will highlight two interesting sequences within APP that likely contribute to this duality in APP function. First, it has been found that caspase-mediated cleavage of APP in the cytosolic region may release a cytotoxic peptide, C31, which plays a role in synapse loss and neuronal death. Second, recent studies have implicated the -YENPTY- motif in the cytoplasmic region as a domain that modulates several APP activities through phosphorylation and dephosphorylation of the first tyrosine residue. Thus, this review summarizes the current understanding of various APP proteolytic products and the interplay among them to gain deeper insights into the possible mechanisms underlying neurodegeneration and AD pathophysiology.
Figures

References
-
- Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slut-sky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12(12):1567–1576. - PubMed
-
- Allinson TM, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res. 2003;74(3):342–352. - PubMed
-
- Almkvist O, Basun H, Wagner SL, Rowe BA, Wahlund LO, Lannfelt L. Cerebrospinal fluid levels of alpha-secretase-cleaved soluble amyloid precursor protein mirror cognition in a Swedish family with Alzheimer disease and a gene mutation. Arch Neurol. 1997;54(5):641–644. - PubMed
-
- Anderson JJ, Holtz G, Baskin PP, Wang R, Mazzarelli L, Wagner SL, Menzaghi F. Reduced cerebrospinal fluid levels of alpha-secretase-cleaved amyloid precursor protein in aged rats: correlation with spatial memory deficits. Neuroscience. 1999;93(4):1409–1420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

