Matrix metalloproteinases and protein tyrosine kinases: potential novel targets in acute lung injury and ARDS
- PMID: 25287998
- PMCID: PMC4188143
- DOI: 10.1378/chest.14-0397
Matrix metalloproteinases and protein tyrosine kinases: potential novel targets in acute lung injury and ARDS
Abstract
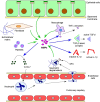
Acute lung injury (ALI) and ARDS fall within a spectrum of pulmonary disease that is characterized by hypoxemia, noncardiogenic pulmonary edema, and dysregulated and excessive inflammation. While mortality rates have improved with the advent of specialized ICUs and lung protective mechanical ventilation strategies, few other therapies have proven effective in the management of ARDS, which remains a significant clinical problem. Further development of biomarkers of disease severity, response to therapy, and prognosis is urgently needed. Several novel pathways have been identified and studied with respect to the pathogenesis of ALI and ARDS that show promise in bridging some of these gaps. This review will focus on the roles of matrix metalloproteinases and protein tyrosine kinases in the pathobiology of ALI in humans, and in animal models and in vitro studies. These molecules can act independently, as well as coordinately, in a feed-forward manner via activation of tyrosine kinase-regulated pathways that are pivotal in the development of ARDS. Specific signaling events involving proteolytic processing by matrix metalloproteinases that contribute to ALI, including cytokine and chemokine activation and release, neutrophil recruitment, transmigration and activation, and disruption of the intact alveolar-capillary barrier, will be explored in the context of these novel molecular pathways.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

