In vivo evidence of pathogenicity of VPS35 mutations in the Drosophila
- PMID: 25288323
- PMCID: PMC4193144
- DOI: 10.1186/s13041-014-0073-y
In vivo evidence of pathogenicity of VPS35 mutations in the Drosophila
Abstract
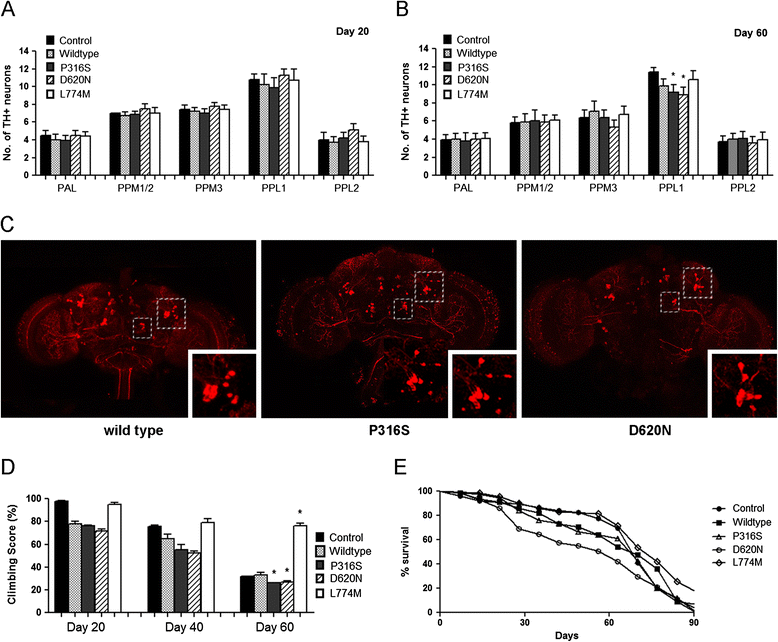
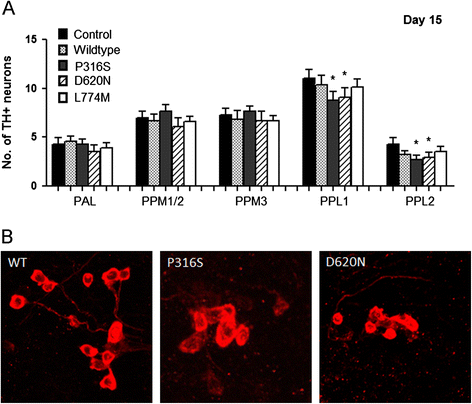
Mutations of VPS35, a component of the retromer complex have been associated with late onset familial Parkinson's disease. The D620N mutation in VPS35 appears to be most prevalent, however, P316S was found in two cases within the same family and a control, whereas L774M was identified in 6 cases and 1 control. In vivo evidence of their pathogenicity is lacking. Here we investigated the in vivo effects of P316S, D620N and L774M using Drosophila as a model. We generated transgenic human VPS35-expressing mutations and demonstrated that VPS35 D620N transgenic flies led to late-onset loss of TH-positive DA neurons, poor mobility, shortened lifespans and increased sensitivity to rotenone, a PD-linked environmental toxin, with some of these phenotypes observed for P316S but not in L774M transgenic flies. We conclude that D620N and to a smaller extent P316S are associated with pathogenicity in PD.
Figures
References
-
- Skinner C, Seaman M, Skinner C, Seaman M. The role of retromer in neurodegenerative disease. In: St. George-Hyslop PH, Mobley WCC, Yves C, editors. Research and Perspectives in Alzheimer’s Disease. Heidelberg: Springer Berlin; 2009. pp. 125–140.
-
- Zimprich A, Benet-Pagès A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC, Lichtner P, Rossle SC, Klopp N, Wolf E, Seppi K, Pirker W, Presslauer S, Mollenhauer B, Katzenschlager R, Foki T, Hotzy C, Reinthaler E, Harutyunyan A, Kralovics R, Peters A, Zimprich F, Brücke T, Poewe W, Auff E, Trenkwalder C, Rost B, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89(1):168–175. doi: 10.1016/j.ajhg.2011.06.008. - DOI - PMC - PubMed
-
- Vilariño-Güell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, Soto-Ortolaza AI, Cobb SA, Wilhoite GJ, Bacon JA, Behrouz B, Melrose HL, Hentati E, Puschmann A, Evans DM, Conibear E, Wasserman WW, Aasly JO, Burkhard PR, Djaldetti R, Ghika J, Hentati F, Krygowska-Wajs A, Lynch T, Melamed E, Rajput A, Rajput AH, Solida A, Wu RM, Uitti RJ, et al. VPS35 mutations in Parkinson disease. Am J Hum Genet. 2011;89(1):162–167. doi: 10.1016/j.ajhg.2011.06.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

