Myeloproliferative neoplasms can be initiated from a single hematopoietic stem cell expressing JAK2-V617F
- PMID: 25288396
- PMCID: PMC4203945
- DOI: 10.1084/jem.20131371
Myeloproliferative neoplasms can be initiated from a single hematopoietic stem cell expressing JAK2-V617F
Abstract
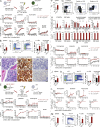
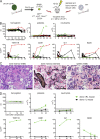
The majority of patients with myeloproliferative neoplasms (MPNs) carry a somatic JAK2-V617F mutation. Because additional mutations can precede JAK2-V617F, it is questioned whether JAK2-V617F alone can initiate MPN. Several mouse models have demonstrated that JAK2-V617F can cause MPN; however, in all these models disease was polyclonal. Conversely, cancer initiates at the single cell level, but attempts to recapitulate single-cell disease initiation in mice have thus far failed. We demonstrate by limiting dilution and single-cell transplantations that MPN disease, manifesting either as erythrocytosis or thrombocytosis, can be initiated clonally from a single cell carrying JAK2-V617F. However, only a subset of mice reconstituted from single hematopoietic stem cells (HSCs) displayed MPN phenotype. Expression of JAK2-V617F in HSCs promoted cell division and increased DNA damage. Higher JAK2-V617F expression correlated with a short-term HSC signature and increased myeloid bias in single-cell gene expression analyses. Lower JAK2-V617F expression in progenitor and stem cells was associated with the capacity to stably engraft in secondary recipients. Furthermore, long-term repopulating capacity was also present in a compartment with intermediate expression levels of lineage markers. Our studies demonstrate that MPN can be initiated from a single HSC and illustrate that JAK2-V617F has complex effects on HSC biology.
© 2014 Lundberg et al.
Figures






References
-
- Baxter, E.J., Scott L.M., Campbell P.J., East C., Fourouclas N., Swanton S., Vassiliou G.S., Bench A.J., Boyd E.M., Curtin N., et al. Cancer Genome Project. 2005. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 365:1054–1061. 10.1016/S0140-6736(05)71142-9 - DOI - PubMed
-
- Beer, P.A., Jones A.V., Bench A.J., Goday-Fernandez A., Boyd E.M., Vaghela K.J., Erber W.N., Odeh B., Wright C., McMullin M.F., et al. 2009. Clonal diversity in the myeloproliferative neoplasms: independent origins of genetically distinct clones. Br. J. Haematol. 144:904–908. 10.1111/j.1365-2141.2008.07560.x - DOI - PubMed
-
- Benz, C., Copley M.R., Kent D.G., Wohrer S., Cortes A., Aghaeepour N., Ma E., Mader H., Rowe K., Day C., et al. 2012. Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell. 10:273–283. 10.1016/j.stem.2012.02.007 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

