Genetic dissection of the vav2-rac1 signaling axis in vascular smooth muscle cells
- PMID: 25288640
- PMCID: PMC4248731
- DOI: 10.1128/MCB.01066-14
Genetic dissection of the vav2-rac1 signaling axis in vascular smooth muscle cells
Abstract
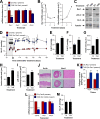
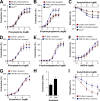
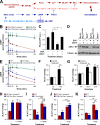
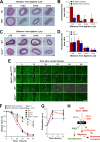
Vascular smooth muscle cells (vSMCs) are key in the regulation of blood pressure and the engagement of vascular pathologies, such as hypertension, arterial remodeling, and neointima formation. The role of the Rac1 GTPase in these cells remains poorly characterized. To clarify this issue, we have utilized genetically engineered mice to manipulate the signaling output of Rac1 in these cells at will using inducible, Cre-loxP-mediated DNA recombination techniques. Here, we show that the expression of an active version of the Rac1 activator Vav2 exclusively in vSMCs leads to hypotension as well as the elimination of the hypertension induced by the systemic loss of wild-type Vav2. Conversely, the specific depletion of Rac1 in vSMCs causes defective nitric oxide vasodilation responses and hypertension. Rac1, but not Vav2, also is important for neointima formation but not for hypertension-driven vascular remodeling. These animals also have allowed us to dismiss etiological connections between hypertension and metabolic disease and, most importantly, identify pathophysiological programs that cooperate in the development and consolidation of hypertensive states caused by local vascular tone dysfunctions. Finally, our results suggest that the therapeutic inhibition of Rac1 will be associated with extensive cardiovascular system-related side effects and identify pharmacological avenues to circumvent them.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G. 2000. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 275:21722–21729. 10.1074/jbc.M000753200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
