Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging
- PMID: 25288763
- PMCID: PMC4210348
- DOI: 10.1073/pnas.1412754111
Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging
Abstract
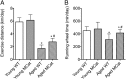
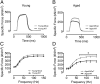
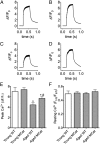
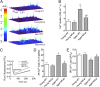
Age-related skeletal muscle dysfunction is a leading cause of morbidity that affects up to half the population aged 80 or greater. Here we tested the effects of increased mitochondrial antioxidant activity on age-dependent skeletal muscle dysfunction using transgenic mice with targeted overexpression of the human catalase gene to mitochondria (MCat mice). Aged MCat mice exhibited improved voluntary exercise, increased skeletal muscle specific force and tetanic Ca(2+) transients, decreased intracellular Ca(2+) leak and increased sarcoplasmic reticulum (SR) Ca(2+) load compared with age-matched wild type (WT) littermates. Furthermore, ryanodine receptor 1 (the sarcoplasmic reticulum Ca(2+) release channel required for skeletal muscle contraction; RyR1) from aged MCat mice was less oxidized, depleted of the channel stabilizing subunit, calstabin1, and displayed increased single channel open probability (Po). Overall, these data indicate a direct role for mitochondrial free radicals in promoting the pathological intracellular Ca(2+) leak that underlies age-dependent loss of skeletal muscle function. This study harbors implications for the development of novel therapeutic strategies, including mitochondria-targeted antioxidants for treatment of mitochondrial myopathies and other healthspan-limiting disorders.
Keywords: aging; exercise capacity; muscle weakness; oxidation; skeletal muscle.
Conflict of interest statement
Conflict of interest statement: A.R.M. is a consultant for ARMGO, which is targeting RyR channels for therapeutic purposes.
Figures

References
-
- Boockvar KS, Meier DE. Palliative care for frail older adults: “There are things I can’t do anymore that I wish I could . . .”. JAMA. 2006;296(18):2245–2253. - PubMed
-
- Roubenoff R, Castaneda C. Sarcopenia-understanding the dynamics of aging muscle. JAMA. 2001;286(10):1230–1231. - PubMed
-
- Sardu C, Marfella R, Santulli G. Impact of diabetes mellitus on the clinical response to cardiac resynchronization therapy in elderly people. J Cardiovasc Transl Res. 2014;7(3):362–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

