LEOPARD syndrome-associated SHP2 mutation confers leanness and protection from diet-induced obesity
- PMID: 25288766
- PMCID: PMC4210352
- DOI: 10.1073/pnas.1406107111
LEOPARD syndrome-associated SHP2 mutation confers leanness and protection from diet-induced obesity
Abstract
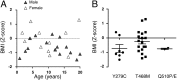
LEOPARD syndrome (multiple Lentigines, Electrocardiographic conduction abnormalities, Ocular hypertelorism, Pulmonary stenosis, Abnormal genitalia, Retardation of growth, sensorineural Deafness; LS), also called Noonan syndrome with multiple lentigines (NSML), is a rare autosomal dominant disorder associating various developmental defects, notably cardiopathies, dysmorphism, and short stature. It is mainly caused by mutations of the PTPN11 gene that catalytically inactivate the tyrosine phosphatase SHP2 (Src-homology 2 domain-containing phosphatase 2). Besides its pleiotropic roles during development, SHP2 plays key functions in energetic metabolism regulation. However, the metabolic outcomes of LS mutations have never been examined. Therefore, we performed an extensive metabolic exploration of an original LS mouse model, expressing the T468M mutation of SHP2, frequently borne by LS patients. Our results reveal that, besides expected symptoms, LS animals display a strong reduction of adiposity and resistance to diet-induced obesity, associated with overall better metabolic profile. We provide evidence that LS mutant expression impairs adipogenesis, triggers energy expenditure, and enhances insulin signaling, three features that can contribute to the lean phenotype of LS mice. Interestingly, chronic treatment of LS mice with low doses of MEK inhibitor, but not rapamycin, resulted in weight and adiposity gains. Importantly, preliminary data in a French cohort of LS patients suggests that most of them have lower-than-average body mass index, associated, for tested patients, with reduced adiposity. Altogether, these findings unravel previously unidentified characteristics for LS, which could represent a metabolic benefit for patients, but may also participate to the development or worsening of some traits of the disease. Beyond LS, they also highlight a protective role of SHP2 global LS-mimicking modulation toward the development of obesity and associated disorders.
Keywords: adipose tissue; energy metabolism; ras/MAPK; rasopathies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
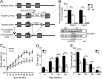
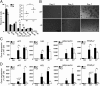
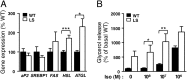


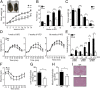
References
-
- Grossmann KS, Rosário M, Birchmeier C, Birchmeier W. The tyrosine phosphatase Shp2 in development and cancer. Adv Cancer Res. 2010;106:53–89. - PubMed
-
- Zhang SQ, et al. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol Cell. 2004;13(3):341–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

