Actin Cytoskeleton and Golgi Involvement in Barley stripe mosaic virus Movement and Cell Wall Localization of Triple Gene Block Proteins
- PMID: 25288925
- PMCID: PMC4174794
- DOI: 10.5423/PPJ.OA.09.2012.0144
Actin Cytoskeleton and Golgi Involvement in Barley stripe mosaic virus Movement and Cell Wall Localization of Triple Gene Block Proteins
Abstract
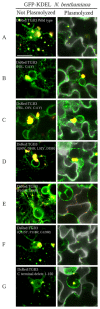
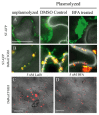
Barley stripe mosaic virus (BSMV) induces massive actin filament thickening at the infection front of infected Nicotiana benthamiana leaves. To determine the mechanisms leading to actin remodeling, fluorescent protein fusions of the BSMV triple gene block (TGB) proteins were coexpressed in cells with the actin marker DsRed: Talin. TGB ectopic expression experiments revealed that TGB3 is a major elicitor of filament thickening, that TGB2 resulted in formation of intermediate DsRed:Talin filaments, and that TGB1 alone had no obvious effects on actin filament structure. Latrunculin B (LatB) treatments retarded BSMV cell-to-cell movement, disrupted actin filament organization, and dramatically decreased the proportion of paired TGB3 foci appearing at the cell wall (CW). BSMV infection of transgenic plants tagged with GFP-KDEL exhibited membrane proliferation and vesicle formation that were especially evident around the nucleus. Similar membrane proliferation occurred in plants expressing TGB2 and/or TGB3, and DsRed: Talin fluorescence in these plants colocalized with the ER vesicles. TGB3 also associated with the Golgi apparatus and overlapped with cortical vesicles appearing at the cell periphery. Brefeldin A treatments disrupted Golgi and also altered vesicles at the CW, but failed to interfere with TGB CW localization. Our results indicate that actin cytoskeleton interactions are important in BSMV cell-to-cell movement and for CW localization of TGB3.
Keywords: Barley stripe mosaic virus; Hordeivirus; Latrunculin B; Membrane proliferation; Triple gene block.
Figures






References
-
- Baluska F, Jasik J, Edelmann HG, Salajova T, Volkmann D. Latrunculin B-induced plant dwarfism: Plant cell elongation is F-actin-dependent. Dev Biol. 2001;231:113–124. - PubMed
-
- Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C. Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J. 1998;15:441–447. - PubMed
-
- Cowan GH, Lioliopoulou F, Ziegler A, Torrance L. Subcellular localisation, protein interactions, and RNA binding of potato mop-top virus triple gene block proteins. Virology. 2002;298:106–115. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

