Biological Control of Meloidogyne hapla Using an Antagonistic Bacterium
- PMID: 25289015
- PMCID: PMC4181115
- DOI: 10.5423/PPJ.OA.02.2014.0013
Biological Control of Meloidogyne hapla Using an Antagonistic Bacterium
Abstract
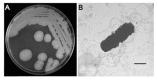
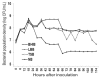
We examined the efficacy of a bacterium for biocontrol of the root-knot nematode (RKN) Meloidogyne hapla in carrot (Daucus carota subsp. sativus) and tomato (Solanum lycopersicum). Among 542 bacterial isolates from various soils and plants, the highest nematode mortality was observed for treatments with isolate C1-7, which was identified as Bacillus cereus based on cultural and morphological characteristics, the Biolog program, and 16S rRNA sequencing analyses. The population density and the nematicidal activity of B. cereus C1-7 remained high until the end of culture in brain heart infusion broth, suggesting that it may have sustainable biocontrol potential. In pot experiments, the biocontrol efficacy of B. cereus C1-7 was high, showing complete inhibition of root gall or egg mass formation by RKN in carrot and tomato plants, and subsequently reducing RKN damage and suppressing nematode population growth, respectively. Light microscopy of RKN-infected carrot root tissues treated with C1-7 showed reduced formation of gall cells and fully developed giant cells, while extensive gall cells and fully mature giant cells with prominent cell wall ingrowths formed in the untreated control plants infected with RKNs. These histopathological characteristics may be the result of residual or systemic biocontrol activity of the bacterium, which may coincide with the biocontrol efficacies of nematodes in pots. These results suggest that B. cereus C1-7 can be used as a biocontrol agent for M. hapla.
Keywords: Bacillus cereus; Meloidogyne hapla; biological control; giant cell.
Figures


References
-
- Barker KR. Nematode extractions and bioassays. In: Barker KR, Carter CC, Sasser JN, editors. An advanced treatise on Meloidogyne, Vol II Methodology. North Carolina State University; Raleigh, NC, USA: 1985. pp. 19–35.
-
- Bélair C. Tolerance of carrot cultivars to northern root-knot nematode as influenced by preplant population densities. Phytoprotection. 1984;65:69–73.
-
- Bonants PJM, Fitters PFL, Thijs H, Belder ED, Waalwijk C, Henfling JWDM. A basic serine protease from Paecilomyces lilacinus with biological activity against Meloidogyne hapla eggs. Microbiol. 1995;141:775–784. - PubMed
-
- Brown EB. Cultural and biological control methods. In: Southey JF, editor. Plant Nematology. 3rd ed. London, UK: Her Majesty’s Stationery Office; 1978. pp. 269–82.
-
- Carneiro RMDG, De Souza IS, Belarmino LC. Nematicidal activity of Bacillus spp. strains on juveniles of Meloidogyne javanica. Nematol Brasileira. 1998;22:12–21.
LinkOut - more resources
Full Text Sources
Other Literature Sources

