Etanercept in the treatment of ankylosing spondylitis: A systematic review and meta-analysis
- PMID: 25289064
- PMCID: PMC4186333
- DOI: 10.3892/etm.2014.1974
Etanercept in the treatment of ankylosing spondylitis: A systematic review and meta-analysis
Abstract
Etanercept (ETN) has been widely applied in the treatment of ankylosing spondylitis (AS). As the use of ETN has increased, associated adverse effects have been reported frequently. Previous meta-analyses have focused on comparing the differences in clinical outcomes between ETN and placebo (PBO). The present meta-analysis evaluated randomised controlled trials (RCTs) to compare the effects of ETN and a PBO or sulfasalazine (SSZ) in patients with AS. The study population characteristics and the main results, including the Assessment in AS 20% response (ASAS 20), the Bath AS Disease Activity Index (BASDAI) and the Bath AS Functional Index (BASFI), were extracted. The pooled odds ratios (ORs) or weighted mean differences (MDs) were calculated using a fixed or random effects model. Fifteen randomised controlled trials (RCTs) involving 2,194 subjects were included. Compared with a PBO, ETN significantly improved the ASAS 20 [P<0.00001; OR, 8.25; 95% confidence interval (CI), 5.92-11.50], BASDAI (P<0.00001; MD, -18.81; 95% CI, -24.47 to -13.15) and BASFI (P<0.00001; standard MD, -0.68; 95% CI, -0.85 to -0.50). In comparison with SSZ, ETN significantly decreased the BASDAI (P<0.00001; MD, -2.40; 95% CI, -2.89 to -1.90) and C-reactive protein (CRP) levels (P<0.0001; MD, -8.01; 95% CI, -11.73 to -4.29). The most common adverse effect of ETN was an injection site reaction. This meta-analysis shows that ETN monotherapy is effective in improving physical function and reducing disease activity in patients with AS. Compared with SSZ, ETN markedly decreased the BASDAI and CRP levels. However, the efficacy of ETN in treating AS requires further evaluation by more RCTs in a larger population of patients prior to recommending ETN as a substitute for synthetic disease-modifying antirheumatic drug (DMARD) monotherapy, or combinations of synthetic DMARDs.
Keywords: ankylosing spondylitis; etanercept; meta-analysis; systematic review.
Figures
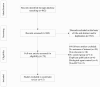

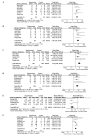


References
-
- Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–1390. - PubMed
-
- Feldtkeller E, Khan M, van der Heijde D, et al. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23:61–66. - PubMed
-
- Sieper J, Braun J. Anti-TNF agents for the treatment of spondyloarthropathies. Expert Opin Emerg Drugs. 2002;7:235–246. - PubMed
-
- Gratacos J, Collado A, Filella X, et al. Serum cytokines (IL-6, TNF-α, IL-1β and IFN-γ) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity. Rheumatology. 1994;33:927–931. - PubMed
-
- Braun J, Bollow M, Neure L, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995;38:499–505. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
