Human neural stem cell transplantation provides long-term restoration of neuronal plasticity in the irradiated hippocampus
- PMID: 25289634
- PMCID: PMC5027839
- DOI: 10.3727/096368914X684600
Human neural stem cell transplantation provides long-term restoration of neuronal plasticity in the irradiated hippocampus
Abstract
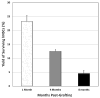
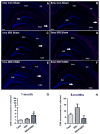
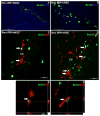
For the majority of CNS malignancies, radiotherapy provides the best option for forestalling tumor growth, but is frequently associated with debilitating and progressive cognitive dysfunction. Despite the recognition of this serious side effect, satisfactory long-term solutions are not currently available and have prompted our efforts to explore the potential therapeutic efficacy of cranial stem cell transplants. We have demonstrated that intrahippocampal transplantation of human neural stem cells (hNSCs) can provide long-lasting cognitive benefits using an athymic rat model subjected to cranial irradiation. To explore the possible mechanisms underlying the capability of engrafted cells to ameliorate radiation-induced cognitive dysfunction we analyzed the expression patterns of the behaviorally induced activity-regulated cytoskeleton-associated protein (Arc) in the hippocampus at 1 and 8 months postgrafting. While immunohistochemical analyses revealed a small fraction (4.5%) of surviving hNSCs in the irradiated brain that did not express neuronal or astroglial makers, hNSC transplantation impacted the irradiated microenvironment of the host brain by promoting the expression of Arc at both time points. Arc is known to play key roles in the neuronal mechanisms underlying long-term synaptic plasticity and memory and provides a reliable marker for detecting neurons that are actively engaged in spatial and contextual information processing associated with memory consolidation. Cranial irradiation significantly reduced the number of pyramidal (CA1) and granule neurons (DG) expressing behaviorally induced Arc at 1 and 8 months postirradiation. Transplantation of hNSCs restored the expression of plasticity-related Arc in the host brain to control levels. These findings suggest that hNSC transplantation promotes the long-term recovery of host hippocampal neurons and indicates that one mechanism promoting the preservation of cognition after irradiation involves trophic support from engrafted cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

