Prostaglandin E2 stimulates β1-integrin expression in hepatocellular carcinoma through the EP1 receptor/PKC/NF-κB pathway
- PMID: 25289898
- PMCID: PMC5377465
- DOI: 10.1038/srep06538
Prostaglandin E2 stimulates β1-integrin expression in hepatocellular carcinoma through the EP1 receptor/PKC/NF-κB pathway
Abstract
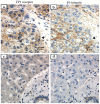
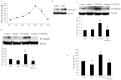
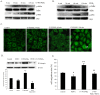
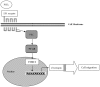
Prostaglandin E2 (PGE2) has been implicated in cell invasion in hepatocellular carcinoma (HCC), via increased β1-integrin expression and cell migration; however, the mechanism remains unclear. PGE2 exerts its effects via four subtypes of the E prostanoid receptor (EP receptor 1-4). The present study investigated the effect of EP1 receptor activation on β1-integrin expression and cell migration in HCC. Cell migration increased by 60% in cells treated with 17-PT-PGE2 (EP1 agonist), which was suppressed by pretreatment with a β1-integrin polyclonal antibody. PGE2 increased β1-integrin expression by approximately 2-fold. EP1 receptor transfection or treatment with 17-PT-PGE2 mimicked the effect of PGE2 treatment. EP1 siRNA blocked PGE2-mediated β1-integrin expression. 17-PT-PGE2 treatment induced PKC and NF-κB activation; PKC and NF-κB inhibitors suppressed 17-PT-PGE2-mediated β1-integrin expression. FoxC2, a β1-integrin transcription factor, was also upregulated by 17-PT-PGE2. NF-κB inhibitor suppressed 17-PT-PGE2-mediated FoxC2 upregulation. Immunohistochemistry showed p65, FoxC2, EP1 receptor and β1-integrin were all highly expressed in the HCC cases. This study suggested that PGE2 upregulates β1-integrin expression and cell migration in HCC cells by activating the PKC/NF-κB signaling pathway. Targeting PGE2/EP1/PKC/NF-κB/FoxC2/β1-integrin pathway may represent a new therapeutic strategy for the prevention and treatment of this cancer.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

