Perphenazine versus low-potency first-generation antipsychotic drugs for schizophrenia
- PMID: 25290157
- PMCID: PMC11055212
- DOI: 10.1002/14651858.CD009369.pub2
Perphenazine versus low-potency first-generation antipsychotic drugs for schizophrenia
Abstract
Background: Antipsychotic drugs are the core treatment for schizophrenia. Treatment guidelines state that there is no difference in efficacy between the various first-generation antipsychotics, however, low-potency first-generation antipsychotic drugs are sometimes perceived as less efficacious than high-potency first-generation compounds by clinicians, and they also seem to differ in their side effects.
Objectives: To review the effects of high-potency, first-generation perphenazine compared with low-potency, first-generation antipsychotic drugs for people with schizophrenia.
Search methods: We searched the Cochrane Schizophrenia Group Trials Register (October 2010).
Selection criteria: We included all randomised controlled trials (RCTs) comparing perphenazine with first-generation, low-potency antipsychotic drugs for people with schizophrenia or schizophrenia-like psychoses.
Data collection and analysis: We extracted data independently. For dichotomous data we calculated risk ratios (RR) and their 95% confidence intervals (CI) on an intention-to-treat basis and using a random-effects model.
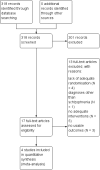
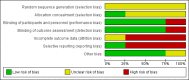
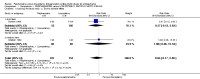
Main results: The review currently includes four relevant randomised trials with 365 participants. The size of the included studies was between 42 and 158 participants with a study length between one and four months. Overall, the methods of sequence generation and allocation concealment were poorly reported. Most studies were rated as low risk of bias in terms of blinding. Overall, attrition bias in the studies was high.The effects of perphenazine and low-potency antipsychotic drugs seemed to be similar in terms of the primary outcome - response to treatment (perphenazine 58%, low-potency antipsychotics 59%, 2 RCTs, n = 138, RR 0.97 CI 0.74 to 1.26 - moderate quality of evidence). There was also no clear evidence of a difference in acceptability of treatment with the number of participants leaving the studies early due to any reason, however results were imprecise (perphenazine 30%, low-potency antipsychotics 28%, 3 RCTs, n = 323, RR 0.78 CI 0.35 to 1.76, very low quality of evidence).There were low numbers of studies available for the outcomes experiencing at least one adverse effect (perphenazine 33%, low-potency antipsychotics 47%, 2 RCTs, n = 165, RR 0.83 CI 0.36 to 1.95, low quality evidence) and experiencing at least one movement disorder (perphenazine 22%, low-potency first-generation antipsychotics 0%, 1 RCT, n = 69, RR 15.62 CI 0.94 to 260.49, low quality evidence), and the confidence intervals for the estimated effects did not exclude important differences. Akathisia was more frequent in the perphenazine group (perphenazine 25%, low-potency antipsychotics 22%, 2 RCTs, n = 227, RR 9.45 CI 1.69 to 52.88), whereas severe toxicity was less so (perphenazine 42%, low-potency antipsychotics 69%, 1 RCT, n = 96, RR 0.61 CI 0.41 to 0.89).There were three deaths in the low-potency group by four months but the difference between groups was not significant (perphenazine 0%, low-potency antipsychotics 2%, 1 RCT, n = 96, RR 0.14 CI 0.01 to 2.69, moderate quality evidence). No data were available for our prespecified outcomes of interest sedation or quality of life. Data were not available for other outcomes such as relapse, service use, costs and satisfaction with care.The event rates reported quote simple aggregates and are not based on the RRs.
Authors' conclusions: The results do not show a superiority in efficacy of high-potency perphenazine compared with low-potency first-generation antipsychotics. There is some evidence that perphenazine is more likely to cause akathisia and less likely to cause severe toxicity, but most adverse effect results were equivocal. The number of studies as well as the quality of studies is low, with quality of evidence for the main outcomes ranging from moderate to very low, so more randomised evidence would be needed for conclusions to be made.
Conflict of interest statement
Magdolna Tardy ‐ none to declare.
Maximilian Huhn ‐ none to declare.
Rolf Engel ‐ none to declare.
Stefan Leucht ‐ has received honoraria for lectures from Abbvie, Astra Zeneca, BristolMyersSquibb, ICON, EliLilly, Janssen, Johnson & Johnson, Roche, SanofiAventis, Lundbeck and Pfizer; honoraria for consulting/advisory boards from Roche, EliLilly, Medavante, BristolMyersSquibb, Alkermes, Janssen, Johnson & Johnson and Lundbeck. EliLilly has provided medication for a study with SL also primary investigator.
Figures











Update of
- doi: 10.1002/14651858.CD009369
References
References to studies included in this review
Adelson 1962 {published data only}
-
- Adelson D, Epstein LJ. A study of phenothiazines with males and female chronically ill schizophrenic patients. Journal of Nervous and Mental Diseases 1962;134(1):543‐54. - PubMed
Hanlon 1965 {published data only}
-
- Hanlon TE, Michaux MH, Ota KY, Shaffer JW, Kurland AA. The comparative effectiveness of eight phenothiazines. Psychopharmacologia 1965;7:89‐106. - PubMed
Kurland 1961 {published data only}
-
- Kurland AA, Hanlon TE, Tatom MH, Ota KY, Simopoulos AM. The comparative effectiveness of six phenothiazine compounds, phenobarbital and inert placebo in the treatment of acutely ill patients: global measures of severity of illness. Journal of Nervous and Mental Disease 1961;133:1‐18. - PubMed
Shalev 1993 {published data only}
-
- Shalev A, Hermesh H, Rothberg J, Munitz H. Poor neuroleptic response in acutely exacerbated schizophrenic patients. Acta Psychiatrica Scandinavica 1993;87(2):86‐91. - PubMed
References to studies excluded from this review
Akimoto 1966 {published data only}
-
- Akimoto H, Shimazaki T, Shibata N, Sato Y, Takahashi R. Current status of pharmacotherapy in schizophrenia. Folia Psychiatrica et Neurologica Japonica 1966;20(1):1‐8. - PubMed
Anon 2006 {published data only}
-
- Anon. A new comparison of antipsychotic drugs. Harvard Mental Health Letter 2006;22(7):7. - PubMed
Bennett 1961 {published data only}
-
- Bennett JL, Kooi KA. Five phenothiazine derivatives. Evaluation and toxicity studies. Archives of General Psychiatry 1961;4:413‐8.
Casey 1960 {published data only}
-
- Casey JF, Lasky JJ, Klett CJ, Hollister LE. Treatment of schizophrenic reactions with phenothiazine derivatives. American Journal of Psychiatry 1960;August 1960:97‐105. - PubMed
Hollister 1974 {published data only}
-
- Hollister LE, Overall JE, Kimbell IJ, Pokorny A. Specific indications for different classes of phenothiazines. Archives of General Psychiatry 1974;30(1):94‐9. - PubMed
Lapolla 1967 {published data only}
-
- Lapolla A. A double‐blind evaluation of chlorpromazine versus a combination of perphenazine and amitriptyline. International Journal of Neuropsychiatry 1967;3(5):403‐5.
Loza 2001 {published data only}
-
- Loza B, Kucharska‐Pietura K, Debowska G. Atypical versus typical antipsychotic treatment prognosis in first‐episode paranoid schizophrenia based on wcst and dichotic listening scores. European Neuropsychopharmacology 2001;11(3):285.
Nordic 1986 {published data only}
-
- Gerlach J. Tardive dyskinesia: pathophysiological mechanisms and clinical trials. L'Encephale 1988;14:227‐32. - PubMed
-
- Nordic Dyskinesia Study Group. Effect of different neuroleptics in tardive dyskinesia and parkinsonism. A video‐controlled multicenter study with chlorprothixene, perphenazine, haloperidol and haloperidol + biperiden. Psychopharmacology 1986;90(4):423‐9. - PubMed
-
- Povlsen UJ, Noring U, Meidahl B, Korsgaard S, Waehrens J, Gerlach J. The effects of neuroleptics on tardive dyskinesias. A video‐controlled, randomized study of chlorprothixene, perphenazine, haloperidol and haloperidol + biperiden [Neuroleptikas virkning pa tardive dyskinesier. En videokontrolleret, randomiseret undersogelse med klorprotixen, perfenazin, haloperidol og haloperidol + biperiden]. Ugeskrift for Laeger 1987;149(25):1682‐5. - PubMed
Schulsinger 1958 {published data only}
-
- Schulsinger F, Jensen R. Trilafon (perphenazine) and largactil (chlorpromazine) in chronic schizophrenia. A comparison [Trilafon (perfenazin) og Largactil (klorpromazin) hos kronisk sindssyge]. Ugeskrift For Laeger 1958;120(12):366‐9. - PubMed
Smith 1959 {published data only}
-
- Smith JA, Christian D, Mansfield E, Figaredo A. A graphic comparison of five phenothiazines. American Journal of Psychiatry 1959;116:392‐9. - PubMed
Svestka 1972 {published data only}
-
- Svestka J, Nahunek K. A comparison of pimozide with perphenazine in the treatment of acute schizophrenic psychoses. Activitas Nervosa Superior 1972;14(2):93‐4. [MEDLINE: ] - PubMed
Svestka 1974 {published data only}
-
- Svestka J, Nahunek K, Rodova A, Ceskova E. A controlled comparison of oxypertine and perphenazine in schizophrenic psychoses. Activitas Nervosa Superior 1974;16(3):165‐6. - PubMed
Vinar 1968 {published data only}
-
- Vinar O. Differences in therapeutic effects of phenothiazine drugs. Agressologie 1968;9(2):315‐7. - PubMed
Additional references
Altman 1996
Andreasen 2010
APA 1980
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM III). Washington DC: American Psychiatric Association, 1980.
Berger 2003
-
- Berger M. Psychische Erkrankungen. Klinik und Therapie. 2nd Edition. München: Urban & Fischer, 2003.
Bland 1997
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. - PubMed
Campbell 2000
-
- Campbell M, Grimshaw J, Steen N. Sample size calculations for cluster randomised trials. Changing Professional Practice in Europe Group (EU BIOMED II Concerted Action). Journal of Health Services Research and Policy 2000;5:12‐6. - PubMed
Carpenter 1994
-
- Carpenter WT, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681‐90. - PubMed
Davis 1974
-
- Davis JM. Overview: maintenance therapy in psychiatry: I. Schizophrenia. American Journal of Psychiatry 1975;132(12):1237‐45. - PubMed
Davis 1989
-
- Davis JM, Barter JT, Kane JM. Antipsychotic drugs. Comprehensive Textbook of Psychiatry. Williams and Wilkins, 1989.
Deeks 2000
-
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town, South Africa. Cape Town, 2000.
Der‐Simonian 1986
-
- Der‐Simonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Divine 1992
-
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. - PubMed
Dold 2012
Egger 1997
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. - PubMed
Falkai 2005
-
- Falkai P, Wobrock T, Lieberman J. World Federation of Societies of Biological Psychiatry (WFSBP) ‐ guidelines for biological treatment of schizophrenia, part 1: Acute treatment of schizophrenia. World Journal of Biological Psychiatry 2005;6:132‐91. - PubMed
Fenton 2007
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. - PubMed
Gaebel 2006
-
- Gaebel W, Falkai P, Weinmann S. Behandlungsleitlinie Schizophrenie. Darmstadt: Steinkopff, 2006.
GRADE 2004 [Computer program]
-
- GRADE Working Group. GRADE Profiler. Version 3.2. GRADE Working Group, 2004.
Gulliford 1999
-
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Haase 1983
-
- Haase HJ. Dosierung der Neuroleptika. Ein Leitfaden für Klinik und Praxis unter besonderer Berücksichtigung psychotisch Kranker. Erlangen: Perimed Fachbuch‐Verlagsgesellschaft, 1983.
Hartung 2005
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org 2011.
Jones 2006
-
- Jones PB, Barnes TRE, Davies L. Randomized controlled trial of the effect on quality of life of second‐ vs first‐generation antipsychotic drugs in schizophrenia ‐ cost utility of the latest antipsychotic drugs in schizophrenia study (CUtLASS 1). Archives of General Psychiatry 2006;63:1079‐86. - PubMed
Kay 1986
-
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda (NY): Multi‐Health Systems, 1986.
Klein 1969
-
- Klein DF, Davis JM. Diagnosis and Drug Treatment of Psychiatric Disorders. Baltimore, MD: Williams and Wilkins, 1969.
Lehman 2004
-
- Lehman AF, Lieberman JA, Dixon LB. Practice guideline for the treatment of patients with schizophrenia, second edition. American Journal of Psychiatry 2004;161:1‐56. - PubMed
Leon 2006
-
- Leon AC, Mallinckrodt CH, Chuang‐Stein C, Archibald DG, Archer GE, Chartier K. Attrition in randomized controlled clinical trials: methodological issues in psychopharmacology. Biological Psychiatry 2006;59:1001‐5. - PubMed
Leucht 2005a
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. What does the PANSS mean?. Schizophrenia Research 2005;79:231‐8. - PubMed
Leucht 2005b
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale Scores. British Journal of Psychiatry 2005;187:366‐71. - PubMed
Leucht 2009
-
- Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second‐generation versus first‐generation antipsychotic drugs for schizophrenia: a meta‐analysis. Lancet 2009;373(9657):31‐41. [PUBMED: 19058842] - PubMed
Lieberman 2005
-
- Lieberman JA, Stroup TS, McEvoy JP. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 2005;353:1209‐23. - PubMed
Lohse 2005
-
- Lohse MJ, Lorenzen A, Müller‐Oerlinghausen B. Psychopharmaka. In: Schwabe U, Pfaffrath D editor(s). Arzneimittelverordnungsreport. Berlin: Springer, 2005:820‐64.
Marshall 2000
-
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. - PubMed
Marvaha 2004
-
- Marwaha S, Johnson S. Schizophrenia and employment ‐ a review. Social Psychiatry and Psychiatric Epidemiology 2004;39:337‐49. - PubMed
Moher 2010
Overall 1962
-
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐12.
Review Manager 2008 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Schünemann 2008
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:359‐83.
Seeman 1975
-
- Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science 1975;188(4194):1217‐9. - PubMed
Tardy 2014
Tardy 2014a
Tardy 2014b
Tardy 2014c
Trikalinos 2004
-
- Trikalinos TA, Churchill R, Ferri M, Leucht S, Tuunainen A, Wahlbeck K, et al. Effect sizes in cumulative meta‐analyses of mental health randomized trials evolved over time. Journal of Clinical Epidemiology 2004;57(11):1124‐30. - PubMed
Tsuang 1978
-
- Tsuang MT. Suicide in schizophrenics, manics, depressives, and surgical controls: a comparison with general population suicide mortality. Archives of General Psychiatry 1978;35:153‐5. - PubMed
Xia 2009
-
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

