Positive allostery in metal ion binding by a cooperatively folded β-peptide bundle
- PMID: 25290247
- PMCID: PMC4210112
- DOI: 10.1021/ja508872q
Positive allostery in metal ion binding by a cooperatively folded β-peptide bundle
Erratum in
-
Correction to "Positive allostery in metal ion binding by a cooperatively folded β-peptide bundle".J Am Chem Soc. 2015 Mar 25;137(11):3994. doi: 10.1021/jacs.5b02565. Epub 2015 Mar 16. J Am Chem Soc. 2015. PMID: 25774681 Free PMC article. No abstract available.
Abstract
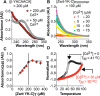
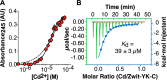
Metal ion binding is exploited by proteins in nature to catalyze reactions, bind molecules, and favor discrete structures, but it has not been demonstrated in β-peptides or their assemblies. Here we report the design, synthesis, and characterization of a β-peptide bundle that uniquely binds two Cd(II) ions in a distinct bicoordinate array. The two Cd(II) ions bind with positive allosteric cooperativity and increase the thermodynamic stability of the bundle by more than 50 °C. This system provides a unique, synthetic context to explore allosteric regulation and should pave the way to sophisticated molecular assemblies with catalytic and substrate-sensing functions that have historically not been available to de novo designed synthetic proteomimetics in water.
Figures


References
-
- Cheng R. P.; DeGrado W. F. J. Am. Chem. Soc. 2001, 123, 5162. - PubMed
- Arvidsson P. I.; Rueping M.; Seebach D. Chem. Comm. 2001, 649.
- Hart S. A.; Bahadoor A. B. F.; Matthews E. E.; Qiu X. Y. J.; Schepartz A. J. Am. Chem. Soc. 2003, 125, 4022–4023. - PubMed
- Kritzer J. A.; Hodsdon M. E.; Schepartz A. J. Am. Chem. Soc. 2005, 127, 4118. - PMC - PubMed
-
- Kritzer J. A.; Lear J. D.; Hodsdon M. E.; Schepartz A. J. Am. Chem. Soc. 2004, 126, 9468. - PubMed
- Michel J.; Harker E. A.; Tirado-Rives J.; Jorgensen W. L.; Schepartz A. J. Am. Chem. Soc. 2009, 131, 6356. - PMC - PubMed
- Bautista A. D.; Appelbaum J. S.; Craig C. J.; Michel J.; Schepartz A. J. Am. Chem. Soc. 2010, 132, 2904. - PMC - PubMed
- Denton E. V.; Craig C. J.; Pongratz R. L.; Appelbaum J. S.; Doerner A. E.; Narayanan A.; Shulman G. I.; Cline G. W.; Schepartz A. Org. Lett. 2013, 15, 5318. - PMC - PubMed
-
- Kritzer J. A.; Luedtke N. W.; Harker E. A.; Schepartz A. J. Am. Chem. Soc. 2005, 127, 14584. - PMC - PubMed
- Kritzer J. A.; Stephens O. M.; Guarracino D. A.; Reznik S. K.; Schepartz A. Bioorg. Med. Chem. 2005, 13, 11. - PMC - PubMed
- Guarracino D. A.; Chiang H. J. R.; Banks T. N.; Lear J. D.; Hodsdon M. E.; Schepartz A. Org. Lett. 2006, 8, 807. - PubMed
- Goodman J. L.; Molski M. A.; Qiu J.; Schepartz A. ChemBioChem 2008, 9, 1576. - PMC - PubMed
- Seebach D.; Gardiner J. Accts, Chem. res. 2008, 41, 1366. - PubMed
- Bautista A. D.; Stephens O. M.; Wang L.; Domaoal R. A.; Anderson K. S.; Schepartz A. Bioorg. Med. Chem. Lett. 2009, 19, 3736. - PMC - PubMed
- Harker E. A.; Daniels D. S.; Guarracino D. A.; Schepartz A. Bioorg. Med. Chem. 2009, 17, 2038. - PMC - PubMed
- Harker E. A.; Schepartz A. ChemBioChem 2009, 10, 990. - PMC - PubMed
- Wang P. S.-P.; Craig C. J.; Schepartz A. Tetrahedron 2012, 68, 4342. - PMC - PubMed
-
- Stephens O. M.; Kim S.; Welch B. D.; Hodsdon M. E.; Kay M. S.; Schepartz A. J. Am. Chem. Soc. 2005, 127, 13126. - PMC - PubMed
- Shandler S. J.; Korendovych I. V.; Moore D. T.; Smith-Dupont K. B.; Streu C. N.; Litvinov R. I.; Billings P. C.; Gai F.; Bennett J. S.; DeGrado W. F. J. Am. Chem. Soc. 2011, 133, 12378. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

