Detection of lipid-linked peptidoglycan precursors by exploiting an unexpected transpeptidase reaction
- PMID: 25291014
- PMCID: PMC4210121
- DOI: 10.1021/ja508147s
Detection of lipid-linked peptidoglycan precursors by exploiting an unexpected transpeptidase reaction
Abstract
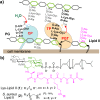
Penicillin-binding proteins (PBPs) are involved in the synthesis and remodeling of bacterial peptidoglycan (PG). Staphylococcus aureus expresses four PBPs. Genetic studies in S. aureus have implicated PBP4 in the formation of highly cross-linked PG, but biochemical studies have not reached a consensus on its primary enzymatic activity. Using synthetic Lipid II, we show here that PBP4 preferentially acts as a transpeptidase (TP) in vitro. Moreover, it is the PBP primarily responsible for incorporating exogenous d-amino acids into cellular PG, implying that it also has TP activity in vivo. Notably, PBP4 efficiently exchanges d-amino acids not only into PG polymers but also into the PG monomers Lipid I and Lipid II. This is the first demonstration that any TP domain of a PBP can activate the PG monomer building blocks. Exploiting the promiscuous TP activity of PBP4, we developed a simple, highly sensitive assay to detect cellular pools of lipid-linked PG precursors, which are of notoriously low abundance. This method, which addresses a longstanding problem, is useful for assessing how genetic and pharmacological perturbations affect precursor levels, and may facilitate studies to elucidate antibiotic mechanism of action.
Figures




References
-
- Sauvage E.; Kerff F.; Terrak M.; Ayala J. A.; Charlier P. FEMS Microbiol. Rev. 2008, 32, 234–258. - PubMed
- Vollmer W.; Blanot D.; de Pedro M. A. FEMS Microbiol. Rev. 2008, 32, 149–167. - PubMed
- Lovering A. L.; Safadi S. S.; Strynadka N. C. J. Annu. Rev. Biochem. 2012, 81, 451–478. - PubMed
- Typas A.; Banzhaf M.; Gross C. A.; Vollmer W. Nat. Rev. Microbiol. 2012, 10, 123–136. - PMC - PubMed
-
- Walsh C.Antibiotics: Actions, Origins, Resistance; ASM Press: Washington, DC, 2003.
-
- Nemmara V. V.; Dzhekieva L.; Sarkar K. S.; Adediran S. A.; Duez C.; Nicholas R. A.; Pratt R. F. Biochemistry 2011, 50, 10091–10101. - PubMed
-
- Bertsche U.; Breukink E.; Kast T.; Vollmer W. J. Biol. Chem. 2005, 280, 38096–38101. - PubMed
- Born P.; Breukink E.; Vollmer W. J. Biol. Chem. 2006, 281, 26985–26993. - PubMed
- Banzhaf M.; van den Berg van Saparoea B.; Terrak M.; Fraipont C.; Egan A.; Philippe J.; Zapun A.; Breukink E.; Nguyen-Distèche M.; den Blaauwen T.; Vollmer W. Mol. Microbiol. 2012, 85, 179–194. - PubMed
- Zapun A.; Philippe J.; Abrahams K. A.; Signor L.; Roper D. I.; Breukink E.; Vernet T. ACS Chem. Biol. 2013, 8, 2688–2696. - PubMed
- Lebar M. D.; Lupoli T. J.; Tsukamoto H.; May J. M.; Walker S.; Kahne D. J. Am. Chem. Soc. 2013, 135, 4632–4635. - PMC - PubMed
- Lebar M. D.; May J. M.; Meeske A. J.; Leiman S. A.; Lupoli T. J.; Tsukamoto H.; Losick R.; Rudner D. Z.; Walker S.; Kahne D. J. Am. Chem. Soc. 2014, 136, 10874–10877. - PMC - PubMed
- For computational modeling of cross-linking:Shi Q.; Meroueh S. O.; Fisher J. F.; Mobashery S. J. Am. Chem. Soc. 2011, 133, 5274–5283. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

