Glucotoxic and diabetic conditions induce caspase 6-mediated degradation of nuclear lamin A in human islets, rodent islets and INS-1 832/13 cells
- PMID: 25292013
- PMCID: PMC4225171
- DOI: 10.1007/s10495-014-1038-4
Glucotoxic and diabetic conditions induce caspase 6-mediated degradation of nuclear lamin A in human islets, rodent islets and INS-1 832/13 cells
Abstract
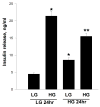
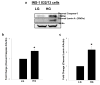
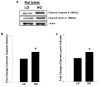
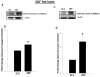
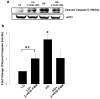
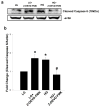
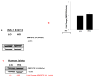
Nuclear lamins form the lamina on the interior surface of the nuclear envelope, and regulate nuclear metabolic events, including DNA replication and organization of chromatin. The current study is aimed at understanding the role of executioner caspase 6 on lamin A integrity in islet β-cells under duress of glucotoxic (20 mM glucose; 24 h) and diabetic conditions. Under glucotoxic conditions, glucose-stimulated insulin secretion and metabolic cell viability were significantly attenuated in INS-1 832/13 cells. Further, exposure of normal human islets, rat islets and INS-1 832/13 cells to glucotoxic conditions leads to caspase 6 activation and lamin A degradation, which is also observed in islets from the Zucker diabetic fatty rat, a model for type 2 diabetes (T2D), and in islets from a human donor with T2D. Z-Val-Glu-Ile-Asp-fluoromethylketone, a specific inhibitor of caspase 6, markedly attenuated high glucose-induced caspase 6 activation and lamin A degradation, confirming that caspase 6 mediates lamin A degradation under high glucose exposure conditions. Moreover, Z-Asp-Glu-Val-Asp-fluoromethylketone, a known caspase 3 inhibitor, significantly inhibited high glucose-induced caspase 6 activation and lamin A degradation, suggesting that activation of caspase 3 might be upstream to caspase 6 activation in the islet β-cell under glucotoxic conditions. Lastly, we report expression of ZMPSTE24, a zinc metallopeptidase involved in the processing of prelamin A to mature lamin A, in INS-1 832/13 cells and human islets; was unaffected by high glucose. We conclude that caspases 3 and 6 could contribute to alterations in the integrity of nuclear lamins leading to metabolic dysregulation and failure of the islet β-cell.
Conflict of interest statement
The authors declare no conflict of interests.
Figures



References
-
- Gruenbaum Y, Wilson KL, Harel A, Goldberg M, Cohen M. Review: nuclear lamins--structural proteins with fundamental functions. J Struct Biol. 2000;129:313–323. - PubMed
-
- Moir RD, Spann TP, Goldman RD. The dynamic properties and possible functions of nuclear lamins. Int Rev Cytol. 1995;162:141–182. - PubMed
-
- Shahzidi S, Brech A, Sioud M, Li X, Suo Z, Nesland JM, Peng Q. Lamin A/C cleavage by caspase-6 activation is crucial for apoptotic induction by photodynamic therapy with hexaminolevulinate in human B-cell lymphoma cells. Cancer Lett. 2013;339:25–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

