Distinct integrin-dependent signals define requirements for lytic granule convergence and polarization in natural killer cells
- PMID: 25292212
- PMCID: PMC4369380
- DOI: 10.1126/scisignal.2005816
Distinct integrin-dependent signals define requirements for lytic granule convergence and polarization in natural killer cells
Abstract
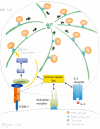
Lytic granules in natural killer (NK) cells represent a dangerous cargo that is targeted for secretion to destroy diseased cells. The appropriate management of these organelles enables the mounting of a precise and valuable host defense. The process of NK cell adhesion to a target cell through engagement of the integrin LFA-1 (lymphocyte function-associated antigen 1) promotes lytic granule organization through complex cellular mechanics and a signaling pathway characterized by Zhang et al. in this issue of Science Signaling. A set of signaling molecules was defined for their ability to promote the polarization of NK cell lytic granules and the microtubule organizing center (MTOC) toward the interface with a target cell. A subset of these signaling molecules was also required for the convergence of lytic granules on the MTOC.
Copyright © 2014, American Association for the Advancement of Science.
Figures

Comment on
-
A signaling network stimulated by β2 integrin promotes the polarization of lytic granules in cytotoxic cells.Sci Signal. 2014 Oct 7;7(346):ra96. doi: 10.1126/scisignal.2005629. Sci Signal. 2014. PMID: 25292215 Free PMC article.
References
-
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. Medline doi:10.1038/ni1582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

