Transferring biomarker into molecular probe: melanin nanoparticle as a naturally active platform for multimodality imaging
- PMID: 25292385
- PMCID: PMC4227813
- DOI: 10.1021/ja505412p
Transferring biomarker into molecular probe: melanin nanoparticle as a naturally active platform for multimodality imaging
Abstract
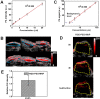
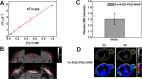
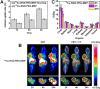
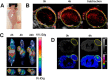
Developing multifunctional and easily prepared nanoplatforms with integrated different modalities is highly challenging for molecular imaging. Here, we report the successful transfer of an important molecular target, melanin, into a novel multimodality imaging nanoplatform. Melanin is abundantly expressed in melanotic melanomas and thus has been actively studied as a target for melanoma imaging. In our work, the multifunctional biopolymer nanoplatform based on ultrasmall (<10 nm) water-soluble melanin nanoparticle (MNP) was developed and showed unique photoacoustic property and natural binding ability with metal ions (for example, (64)Cu(2+), Fe(3+)). Therefore, MNP can serve not only as a photoacoustic contrast agent, but also as a nanoplatform for positron emission tomography (PET) and magnetic resonance imaging (MRI). Traditional passive nanoplatforms require complicated and time-consuming processes for prebuilding reporting moieties or chemical modifications using active groups to integrate different contrast properties into one entity. In comparison, utilizing functional biomarker melanin can greatly simplify the building process. We further conjugated αvβ3 integrins, cyclic c(RGDfC) peptide, to MNPs to allow for U87MG tumor accumulation due to its targeting property combined with the enhanced permeability and retention (EPR) effect. The multimodal properties of MNPs demonstrate the high potential of endogenous materials with multifunctions as nanoplatforms for molecular theranostics and clinical translation.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

