Eph receptors and ephrins: therapeutic opportunities
- PMID: 25292427
- PMCID: PMC4388660
- DOI: 10.1146/annurev-pharmtox-011112-140226
Eph receptors and ephrins: therapeutic opportunities
Abstract
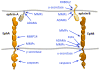
The erythropoietin-producing hepatocellular carcinoma (Eph) receptor tyrosine kinase family plays important roles in developmental processes, adult tissue homeostasis, and various diseases. Interaction with Eph receptor-interacting protein (ephrin) ligands on the surface of neighboring cells triggers Eph receptor kinase-dependent signaling. The ephrins can also transmit signals, leading to bidirectional cell contact-dependent communication. Moreover, Eph receptors and ephrins can function independently of each other through interplay with other signaling systems. Given their involvement in many pathological conditions ranging from neurological disorders to cancer and viral infections, Eph receptors and ephrins are increasingly recognized as attractive therapeutic targets, and various strategies are being explored to modulate their expression and function. Eph receptor/ephrin upregulation in cancer cells, the angiogenic vasculature, and injured or diseased tissues also offer opportunities for Eph/ephrin-based targeted drug delivery and imaging. Thus, despite the challenges presented by the complex biology of the Eph receptor/ephrin system, exciting possibilities exist for therapies exploiting these molecules.
Keywords: angiogenesis; cancer; neurological disease; tyrosine kinase; viral infection.
Figures
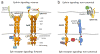
References
-
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–75. - PubMed
-
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. - PubMed
-
- Goldshmit Y, McLenachan S, Turnley A. Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res Brain Res Rev. 2006;52:327–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

