Efficient generation of knock-in transgenic zebrafish carrying reporter/driver genes by CRISPR/Cas9-mediated genome engineering
- PMID: 25293390
- PMCID: PMC4189020
- DOI: 10.1038/srep06545
Efficient generation of knock-in transgenic zebrafish carrying reporter/driver genes by CRISPR/Cas9-mediated genome engineering
Abstract
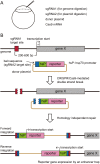
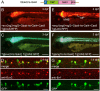
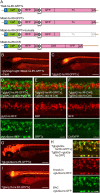
The type II bacterial CRISPR/Cas9 system is rapidly becoming popular for genome-engineering due to its simplicity, flexibility, and high efficiency. Recently, targeted knock-in of a long DNA fragment via homology-independent DNA repair has been achieved in zebrafish using CRISPR/Cas9 system. This raised the possibility that knock-in transgenic zebrafish could be efficiently generated using CRISPR/Cas9. However, how widely this method can be applied for the targeting integration of foreign genes into endogenous genomic loci is unclear. Here, we report efficient generation of knock-in transgenic zebrafish that have cell-type specific Gal4 or reporter gene expression. A donor plasmid containing a heat-shock promoter was co-injected with a short guide RNA (sgRNA) targeted for genome digestion, a sgRNA targeted for donor plasmid digestion, and Cas9 mRNA. We have succeeded in establishing stable knock-in transgenic fish with several different constructs for 4 genetic loci at a frequency being exceeding 25%. Due to its simplicity, design flexibility, and high efficiency, we propose that CRISPR/Cas9-mediated knock-in will become a standard method for the generation transgenic zebrafish.
Figures
References
-
- Higashijima S., Okamoto H., Ueno N., Hotta Y. & Eguchi G. High-frequency generation of transgenic zebrafish which reliably express GFP in whole muscles or the whole body by using promoters of zebrafish origin. Dev Biol 192, 289–299 (1997). - PubMed
-
- Long Q. et al. GATA-1 expression pattern can be recapitulated in living transgenic zebrafish using GFP reporter gene. Development 124, 4105–4111 (1997). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

