Pathways of cholesterol homeostasis in mouse retina responsive to dietary and pharmacologic treatments
- PMID: 25293590
- PMCID: PMC4274074
- DOI: 10.1194/jlr.M053439
Pathways of cholesterol homeostasis in mouse retina responsive to dietary and pharmacologic treatments
Abstract
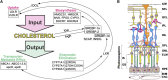
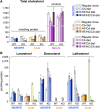
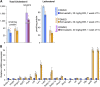
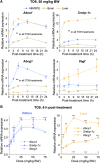
Effects of serum cholesterol on cholesterol content in the retina are currently unknown. It is also unclear how cholesterol levels are controlled in the retina. High-cholesterol diet and oral administrations of simvastatin were used to modulate serum cholesterol in mice. These treatments only modestly affected cholesterol content in the retina and had no significant effect on retinal expression of the major cholesterol- and vision-related genes; the sterol-regulatory element binding protein pathway of transcriptional regulation does not seem to be operative in the retina under the experimental conditions used. Evidence is obtained that posttranslational mechanisms play a role in the control of retinal cholesterol. Retinal genes were only upregulated by oral administrations of TO901317 activating liver X receptors. Three of the upregulated genes could be of particular importance (apoD, Idol, and Rpe65) and have not yet been considered in the context of cholesterol homeostasis in the retina. Collectively, the data obtained identify specific features of retinal cholesterol maintenance and suggest additional therapies for age-related macular degeneration, a blinding disease characterized by cholesterol and lipid accumulations in chorioretinal tissues.
Keywords: 3-hydroxy-3-methyl-glutaryl-CoA reductase; RPE65; age-related macular degeneration; cytochrome P450; liver X receptor; posttranslational regulation; sterol-regulatory element binding protein; transcriptional regulation.
Copyright © 2015 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures






Comment in
-
Cholesterol homeostasis in the retina: seeing is believing.J Lipid Res. 2015 Jan;56(1):1-4. doi: 10.1194/jlr.C056358. Epub 2014 Nov 24. J Lipid Res. 2015. PMID: 25421059 Free PMC article. No abstract available.
References
-
- Repa J. J., Mangelsdorf D. J. 2000. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu. Rev. Cell Dev. Biol. 16: 459–481. - PubMed
-
- Yang C., McDonald J. G., Patel A., Zhang Y., Umetani M., Xu F., Westover E. J., Covey D. F., Mangelsdorf D. J., Cohen J. C., et al. 2006. Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J. Biol. Chem. 281: 27816–27826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

