Arabidopsis lipins, PDAT1 acyltransferase, and SDP1 triacylglycerol lipase synergistically direct fatty acids toward β-oxidation, thereby maintaining membrane lipid homeostasis
- PMID: 25293755
- PMCID: PMC4247580
- DOI: 10.1105/tpc.114.130377
Arabidopsis lipins, PDAT1 acyltransferase, and SDP1 triacylglycerol lipase synergistically direct fatty acids toward β-oxidation, thereby maintaining membrane lipid homeostasis
Abstract
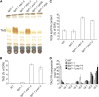
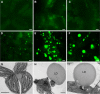
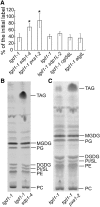
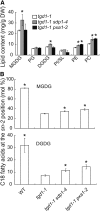
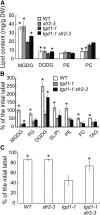
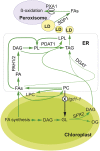
Triacylglycerol (TAG) metabolism is a key aspect of intracellular lipid homeostasis in yeast and mammals, but its role in vegetative tissues of plants remains poorly defined. We previously reported that PHOSPHOLIPID:DIACYLGLYCEROL ACYLTRANSFERASE1 (PDAT1) is crucial for diverting fatty acids (FAs) from membrane lipid synthesis to TAG and thereby protecting against FA-induced cell death in leaves. Here, we show that overexpression of PDAT1 enhances the turnover of FAs in leaf lipids. Using the trigalactosyldiacylglycerol1-1 (tgd1-1) mutant, which displays substantially enhanced PDAT1-mediated TAG synthesis, we demonstrate that disruption of SUGAR-DEPENDENT1 (SDP1) TAG lipase or PEROXISOMAL TRANSPORTER1 (PXA1) severely decreases FA turnover, leading to increases in leaf TAG accumulation, to 9% of dry weight, and in total leaf lipid, by 3-fold. The membrane lipid composition of tgd1-1 sdp1-4 and tgd1-1 pxa1-2 double mutants is altered, and their growth and development are compromised. We also show that two Arabidopsis thaliana lipin homologs provide most of the diacylglycerol for TAG synthesis and that loss of their functions markedly reduces TAG content, but with only minor impact on eukaryotic galactolipid synthesis. Collectively, these results show that Arabidopsis lipins, along with PDAT1 and SDP1, function synergistically in directing FAs toward peroxisomal β-oxidation via TAG intermediates, thereby maintaining membrane lipid homeostasis in leaves.
© 2014 American Society of Plant Biologists. All rights reserved.
Figures




Comment in
-
Lipids in leaves: fatty acid β-oxidation affects lipid homeostasis.Plant Cell. 2014 Oct;26(10):3827. doi: 10.1105/tpc.114.133447. Epub 2014 Oct 28. Plant Cell. 2014. PMID: 25351489 Free PMC article. No abstract available.
Similar articles
-
Dual role for phospholipid:diacylglycerol acyltransferase: enhancing fatty acid synthesis and diverting fatty acids from membrane lipids to triacylglycerol in Arabidopsis leaves.Plant Cell. 2013 Sep;25(9):3506-18. doi: 10.1105/tpc.113.117358. Epub 2013 Sep 27. Plant Cell. 2013. PMID: 24076979 Free PMC article.
-
Phospholipid:diacylglycerol acyltransferase-mediated triacylglycerol biosynthesis is crucial for protection against fatty acid-induced cell death in growing tissues of Arabidopsis.Plant J. 2013 Dec;76(6):930-42. doi: 10.1111/tpj.12343. Epub 2013 Nov 5. Plant J. 2013. PMID: 24118513
-
A Central Role for Triacylglycerol in Membrane Lipid Breakdown, Fatty Acid β-Oxidation, and Plant Survival under Extended Darkness.Plant Physiol. 2017 Jul;174(3):1517-1530. doi: 10.1104/pp.17.00653. Epub 2017 Jun 1. Plant Physiol. 2017. PMID: 28572457 Free PMC article.
-
Lipid turnover during senescence.Plant Sci. 2013 May;205-206:13-9. doi: 10.1016/j.plantsci.2013.01.004. Epub 2013 Jan 26. Plant Sci. 2013. PMID: 23498858 Review.
-
How lipid droplets "TAG" along: Glycerolipid synthetic enzymes and lipid storage.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Oct;1862(10 Pt B):1131-1145. doi: 10.1016/j.bbalip.2017.06.010. Epub 2017 Jun 20. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 28642195 Free PMC article. Review.
Cited by
-
Metabolic engineering of energycane to hyperaccumulate lipids in vegetative biomass.BMC Biotechnol. 2022 Aug 30;22(1):24. doi: 10.1186/s12896-022-00753-7. BMC Biotechnol. 2022. PMID: 36042455 Free PMC article.
-
Integrated lipidomic and transcriptomic analysis reveals diacylglycerol accumulation in olive of Longnan (China).PeerJ. 2023 Aug 11;11:e15724. doi: 10.7717/peerj.15724. eCollection 2023. PeerJ. 2023. PMID: 37583911 Free PMC article.
-
Melatonin-Stimulated Triacylglycerol Breakdown and Energy Turnover under Salinity Stress Contributes to the Maintenance of Plasma Membrane H+-ATPase Activity and K+/Na+ Homeostasis in Sweet Potato.Front Plant Sci. 2018 Feb 27;9:256. doi: 10.3389/fpls.2018.00256. eCollection 2018. Front Plant Sci. 2018. PMID: 29535758 Free PMC article.
-
Genetic enhancement of oil content in potato tuber (Solanum tuberosum L.) through an integrated metabolic engineering strategy.Plant Biotechnol J. 2017 Jan;15(1):56-67. doi: 10.1111/pbi.12590. Epub 2016 Jul 11. Plant Biotechnol J. 2017. PMID: 27307093 Free PMC article.
-
Rice lipases: a conundrum in rice bran stabilization: a review on their impact and biotechnological interventions.Physiol Mol Biol Plants. 2023 Jul;29(7):985-1003. doi: 10.1007/s12298-023-01343-3. Epub 2023 Aug 23. Physiol Mol Biol Plants. 2023. PMID: 37649880 Free PMC article. Review.
References
-
- Alonso J.M., et al. . . (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. - PubMed
-
- Andersson M.X., Goksör M., Sandelius A.S. (2007). Optical manipulation reveals strong attracting forces at membrane contact sites between endoplasmic reticulum and chloroplasts. J. Biol. Chem. 282: 1170–1174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

