Pneumolysin activates macrophage lysosomal membrane permeabilization and executes apoptosis by distinct mechanisms without membrane pore formation
- PMID: 25293758
- PMCID: PMC4196231
- DOI: 10.1128/mBio.01710-14
Pneumolysin activates macrophage lysosomal membrane permeabilization and executes apoptosis by distinct mechanisms without membrane pore formation
Abstract
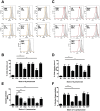
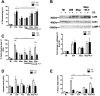
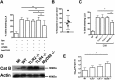
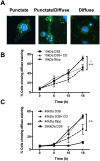
Intracellular killing of Streptococcus pneumoniae is complemented by induction of macrophage apoptosis. Here, we show that the toxin pneumolysin (PLY) contributes both to lysosomal/phagolysosomal membrane permeabilization (LMP), an upstream event programing susceptibility to apoptosis, and to apoptosis execution via a mitochondrial pathway, through distinct mechanisms. PLY is necessary but not sufficient for the maximal induction of LMP and apoptosis. PLY's ability to induce both LMP and apoptosis is independent of its ability to form cytolytic pores and requires only the first three domains of PLY. LMP involves TLR (Toll-like receptor) but not NLRP3/ASC (nucleotide-binding oligomerization domain [Nod]-like receptor family, pyrin domain-containing protein 3/apoptosis-associated speck-like protein containing a caspase recruitment domain) signaling and is part of a PLY-dependent but phagocytosis-independent host response that includes the production of cytokines, including interleukin-1 beta (IL-1β). LMP involves progressive and selective permeability to 40-kDa but not to 250-kDa fluorescein isothiocyanate (FITC)-labeled dextran, as PLY accumulates in the cytoplasm. In contrast, the PLY-dependent execution of apoptosis requires phagocytosis and is part of a host response to intracellular bacteria that also includes NO generation. In cells challenged with PLY-deficient bacteria, reconstitution of LMP using the lysomotrophic detergent LeuLeuOMe favored cell necrosis whereas PLY reconstituted apoptosis. The results suggest that PLY contributes to macrophage activation and cytokine production but also engages LMP. Following bacterial phagocytosis, PLY triggers apoptosis and prevents macrophage necrosis as a component of a broad-based antimicrobial strategy. This illustrates how a key virulence factor can become the focus of a multilayered and coordinated innate response by macrophages, optimizing pathogen clearance and limiting inflammation. Importance: Streptococcus pneumoniae, the commonest cause of bacterial pneumonia, expresses the toxin pneumolysin, which can make holes in cell surfaces, causing tissue damage. Macrophages, resident immune cells essential for responses to bacteria in tissues, activate a program of cell suicide called apoptosis, maximizing bacterial clearance and limiting harmful inflammation. We examined pneumolysin's role in activating this response. We demonstrate that pneumolysin did not directly form holes in cells to trigger apoptosis and show that pneumolysin has two distinct roles which require only part of the molecule. Pneumolysin and other bacterial factors released by bacteria that have not been eaten by macrophages activate macrophages to release inflammatory factors but also make the cell compartment containing ingested bacteria leaky. Once inside the cell, pneumolysin ensures that the bacteria activate macrophage apoptosis, rather than necrosis, enhancing bacterial killing and limiting inflammation. This dual response to pneumolysin is critical for an effective immune response to S. pneumoniae.
Copyright © 2014 Bewley et al.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

