The role of extended-release niacin on immune activation and neurocognition in HIV-infected patients treated with antiretroviral therapy - CTN PT006: study protocol for a randomized controlled trial
- PMID: 25293882
- PMCID: PMC4283109
- DOI: 10.1186/1745-6215-15-390
The role of extended-release niacin on immune activation and neurocognition in HIV-infected patients treated with antiretroviral therapy - CTN PT006: study protocol for a randomized controlled trial
Abstract
Background: Approximately 30% of HIV-1-infected patients receiving antiretroviral therapy who achieve virologic control have unsatisfactory immune reconstitution, with CD4+ T-cell counts persistently below 350 cells/μL. These patients are at elevated risk for clinical progression to AIDS and non-AIDS events. CD4+ T-cell depletion following infection and persistent immune activation can partially explain this low CD4+ T-cell recovery. Recent data suggest a link between the tryptophan oxidation pathway, immune activation and HIV disease progression based on overstimulation of the tryptophan oxidation pathway by HIV antigens and by interferon-gamma. This overstimulation reduces levels of circulating tryptophan, resulting in inflammation which has been implicated in the development of neurocognitive dysfunction. Niacin (vitamin B3) is able to control the excess tryptophan oxidation, correcting tryptophan depletion, and therefore represents an interesting strategy to improve CD4 recovery.We aim to design a crossover proof-of-concept study to assess supplementation with an extended-release form of niacin (Niaspan FCT™) in combination with antiretroviral therapy, compared to antiretroviral therapy alone, on T-cell immune activation as defined by changes in the percentage of CD8+ CD38+ HLA-DR+ T-cells.
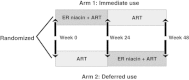
Methods/design: This randomized, open-label, interventional crossover study with an immediate versus deferred use of Niaspan FCT for 24 weeks will assess its ability to reduce immune activation and thus increase CD4 recovery in 20 HIV-infected individuals with suboptimal immune responses despite sustained virologic suppression. A substudy evaluating neurocognitive function will also be conducted.
Discussion: This randomized trial will provide an opportunity to evaluate the potential benefit of oral extended-release niacin, a drug that can indirectly increase tryptophan, to reduce immune activation and in turn increase CD4+ T-cell recovery. The study will also allow for the evaluation of the impact of Niaspan FCT on neurocognitive function in HIV-infected individuals with suboptimal immune responses despite sustained virologic suppression.
Trial registration: This study was registered with ClinicalTrials.gov on 17 December 2013 (registration number: NCT02018965).
Figures
Similar articles
-
Role of in vitro stimulation with lipopolysaccharide on T-cell activation in HIV-infected antiretroviral-treated patients.Clin Dev Immunol. 2012;2012:935425. doi: 10.1155/2012/935425. Epub 2012 Feb 15. Clin Dev Immunol. 2012. PMID: 22400042 Free PMC article.
-
Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy.J Infect Dis. 2013 Jul 15;208(2):249-59. doi: 10.1093/infdis/jit147. Epub 2013 Apr 4. J Infect Dis. 2013. PMID: 23559466 Free PMC article.
-
Impact of extended-release niacin on immune activation in HIV-infected immunological non-responders on effective antiretroviral therapy.HIV Res Clin Pract. 2020 Dec;21(6):182-190. doi: 10.1080/25787489.2020.1866846. Epub 2021 Jan 6. HIV Res Clin Pract. 2020. PMID: 33403940 Clinical Trial.
-
The role of cytokines in the pathogenesis and treatment of HIV infection.Cytokine Growth Factor Rev. 2012 Aug-Oct;23(4-5):207-14. doi: 10.1016/j.cytogfr.2012.05.007. Epub 2012 Jun 26. Cytokine Growth Factor Rev. 2012. PMID: 22738931 Free PMC article. Review.
-
Serious Non-AIDS Events: Therapeutic Targets of Immune Activation and Chronic Inflammation in HIV Infection.Drugs. 2016 Apr;76(5):533-49. doi: 10.1007/s40265-016-0546-7. Drugs. 2016. PMID: 26915027 Free PMC article. Review.
Cited by
-
Oral cannabinoids in people living with HIV on effective antiretroviral therapy: CTN PT028-study protocol for a pilot randomised trial to assess safety, tolerability and effect on immune activation.BMJ Open. 2019 Jan 17;9(1):e024793. doi: 10.1136/bmjopen-2018-024793. BMJ Open. 2019. PMID: 30659041 Free PMC article.
-
Immunometabolism is a key factor for the persistent spontaneous elite control of HIV-1 infection.EBioMedicine. 2019 Apr;42:86-96. doi: 10.1016/j.ebiom.2019.03.004. Epub 2019 Mar 14. EBioMedicine. 2019. PMID: 30879922 Free PMC article.
-
HIV-Related Immune Activation and Inflammation: Current Understanding and Strategies.J Immunol Res. 2021 Sep 29;2021:7316456. doi: 10.1155/2021/7316456. eCollection 2021. J Immunol Res. 2021. PMID: 34631899 Free PMC article. Review.
-
Micronutrient supplementation in adults with HIV infection.Cochrane Database Syst Rev. 2017 May 18;5(5):CD003650. doi: 10.1002/14651858.CD003650.pub4. Cochrane Database Syst Rev. 2017. PMID: 28518221 Free PMC article.
-
Pellagra in isoniazid preventive and antiretroviral therapy.IDCases. 2019 May 3;17:e00550. doi: 10.1016/j.idcr.2019.e00550. eCollection 2019. IDCases. 2019. PMID: 31193074 Free PMC article.
References
-
- Gazzola L, Tincati C, Bellistri GM, Monforte A, Marchetti G. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis. 2009;48:328–337. doi: 10.1086/595851. - DOI - PubMed
-
- Baker JV, Peng G, Rapkin J, Krason D, Reilly C, Cavert WP, Abrams DI, MacArthur RD, Henry K, Neaton JD. Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non-AIDS diseases. J Acquir Immune Defic Syndr. 2008;48:541–546. doi: 10.1097/QAI.0b013e31817bebb3. - DOI - PMC - PubMed
-
- El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, Cohn D, Cooper D, Darbyshire J, Emery S, Fatkenheuer G, Gazzard B, Grund B, Hoy J, Klingman K, Losso M, Markowitz N, Neuhaus J, Phillips A, Rappoport C. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials