AGXT2: a promiscuous aminotransferase
- PMID: 25294000
- PMCID: PMC4254113
- DOI: 10.1016/j.tips.2014.09.005
AGXT2: a promiscuous aminotransferase
Abstract
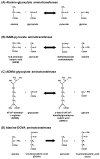
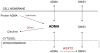
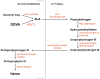
Alanine-glyoxylate aminotransferase 2 (AGXT2) is a multifunctional mitochondrial aminotransferase that was first identified in 1978. The physiological importance of AGXT2 was largely overlooked for three decades because AGXT2 is less active in glyoxylate metabolism than AGXT1, the enzyme that is deficient in primary hyperoxaluria type I. Recently, several novel functions of AGXT2 have been 'rediscovered' in the setting of modern genomic and metabolomic studies. It is now apparent that AGXT2 has multiple substrates and products and that altered AGXT2 activity may contribute to the pathogenesis of cardiovascular, renal, neurological, and hematological diseases. This article reviews the biochemical properties and physiological functions of AGXT2, its unique role at the intersection of key mitochondrial pathways, and its potential as a drug target.
Keywords: alanine; amino acid metabolism; aminotransferase; glyoxylate; methylarginine.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
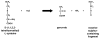

References
-
- Kontani Y, et al. Identity of D-3-aminoisobutyrate-pyruvate aminotransferase with alanine-glyoxylate aminotransferase 2. Biochim Biophys Acta. 1993;1156:161–166. - PubMed
-
- Tamaki N, et al. Purification, characterization and inhibition of D-3-aminoisobutyrate aminotransferase from the rat liver. Eur J Biochem. 1990;189:39–45. - PubMed
-
- Kakimoto Y, et al. D-beta-aminoisobutyrate:pyruvate aminotransferase in mammalian liver and excretion of beta-aminoisobutyrate by man. J Biol Chem. 1969;244:335–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

