Cellular origin and regulation of D- and L-serine in in vitro and in vivo models of cerebral ischemia
- PMID: 25294127
- PMCID: PMC4269747
- DOI: 10.1038/jcbfm.2014.164
Cellular origin and regulation of D- and L-serine in in vitro and in vivo models of cerebral ischemia
Abstract
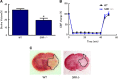
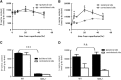
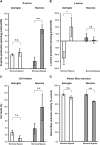
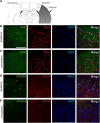
D-Serine is known to be essential for the activation of the N-methyl-D-aspartate (NMDA) receptor in the excitation of glutamatergic neurons, which have critical roles in long-term potentiation and memory formation. D-Serine is also thought to be involved in NMDA receptor-mediated neurotoxicity. The deletion of serine racemase (SRR), which synthesizes D-serine from L-serine, was recently reported to improve ischemic damage in mouse middle cerebral artery occlusion model. However, the cell type in which this phenomenon originates and the regulatory mechanism for D-/L-serine remain elusive. The D-/L-serine content in ischemic brain increased until 20 hours after recanalization and then leveled off gradually. The results of in vitro experiments using cultured cells suggested that D-serine is derived from neurons, while L-serine seems to be released from astroglia. Immunohistochemistry studies of brain tissue after cerebral ischemia showed that SRR is expressed in neurons, and 3-phosphoglycerate dehydrogenase (3-PGDH), which synthesizes L-serine from 3-phosphoglycerate, is located in astrocytes, supporting the results of the in vitro experiments. A western blot analysis showed that neither SRR nor 3-PGDH was upregulated after cerebral ischemia. Therefore, the increase in D-/L-serine was not related to an increase in SRR or 3-PGDH, but to an increase in the substrates of SRR and 3-PGDH.
Figures

References
-
- Itoh Y, Takaoka R, Ohira M, Abe T, Tanahashi N, Suzuki N. Reactive oxygen species generated by mitochondrial injury in human brain microvessel endothelial cells. Clin Hemorheol Microcirc. 2006;34:163–168. - PubMed
-
- Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. - PubMed
-
- Grotta J, Clark W, Coull B, Pettigrew LC, Mackay B, Goldstein LB, et al. Safety and tolerability of the glutamate antagonist CGS 19755 (Selfotel) in patients with acute ischemic stroke. Results of a phase IIa randomized trial. Stroke. 1995;26:602–605. - PubMed
-
- Davis SM, Albers GW, Diener HC, Lees KR, Norris J. Termination of acute stroke studies involving selfotel treatment. ASSIST steering committed. Lancet. 1997;349:32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

