Non-natural olefin cyclopropanation catalyzed by diverse cytochrome P450s and other hemoproteins
- PMID: 25294253
- PMCID: PMC4287214
- DOI: 10.1002/cbic.201402286
Non-natural olefin cyclopropanation catalyzed by diverse cytochrome P450s and other hemoproteins
Abstract
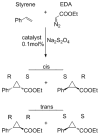
Recent work has shown that engineered variants of cytochrome P450BM3 (CYP102A1) efficiently catalyze non-natural reactions, including carbene and nitrene transfer reactions. Given the broad substrate range of natural P450 enzymes, we set out to explore if this diversity could be leveraged to generate a broad panel of new catalysts for olefin cyclopropanation (i.e., carbene transfer). Here, we took a step towards this goal by characterizing the carbene transfer activities of four new wild-type P450s that have different native substrates. All four were active and exhibited a range of product selectivities in the model reaction: cyclopropanation of styrene by using ethyl diazoacetate (EDA). Previous work on P450BM3 demonstrated that mutation of the axial coordinating cysteine, universally conserved among P450 enzymes, to a serine residue, increased activity for this non-natural reaction. The equivalent mutation in the selected P450s was found to activate carbene transfer chemistry both in vitro and in vivo. Furthermore, serum albumins complexed with hemin were also found to be efficient in vitro cyclopropanation catalysts.
Keywords: cyclopropanation; cytochromes; enzyme catalysis; metalloenzyme.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources