Needle-free injection of insulin powder: delivery efficiency and skin irritation assessment
- PMID: 25294378
- PMCID: PMC4201317
- DOI: 10.1631/jzus.B1400065
Needle-free injection of insulin powder: delivery efficiency and skin irritation assessment
Abstract
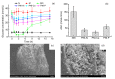
Insulin is widely used in treating diabetes, but still needs to be administered by needle injection. This study investigated a new needle-free approach for insulin delivery. A portable powder needleless injection (PNI) device with an automatic mechanical unit was designed. Its efficiency in delivering insulin was evaluated in alloxan-induced diabetic rabbits. The skin irritation caused by the device was investigated and the results were analyzed in relation to aerodynamic parameters. Inorganic salt-carried insulin powders had hypoglycemic effects, while raw insulin powders were not effective when delivered by PNI, indicating that salt carriers play an important role in the delivery of insulin via PNI. The relative delivery efficiency of phosphate-carried insulin powder using the PNI device was 72.25%. A safety assessment test showed that three key factors (gas pressure, cylinder volume, and nozzle distance) were related to the amount of skin irritation caused by the PNI device. Optimized injection conditions caused minimal skin lesions and are safe to use in practice. The results suggest that PNI has promising prospects as a novel technology for delivering insulin and other biological drugs.
Keywords: Insulin; Powder needleless injection; Skin irritation; Transdermal drug delivery.
Conflict of interest statement
All institutional and national guidelines for the care and use of laboratory animals were followed.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

