Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models
- PMID: 25295214
- PMCID: PMC4177084
- DOI: 10.1155/2014/638747
Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models
Abstract
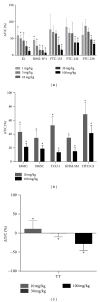
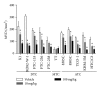
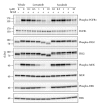
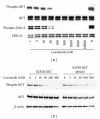
Inhibition of tumor angiogenesis by blockading the vascular endothelial growth factor (VEGF) signaling pathway is a promising therapeutic strategy for thyroid cancer. Lenvatinib mesilate (lenvatinib) is a potent inhibitor of VEGF receptors (VEGFR1-3) and other prooncogenic and prooncogenic receptor tyrosine kinases, including fibroblast growth factor receptors (FGFR1-4), platelet derived growth factor receptor α (PDGFRα), KIT, and RET. We examined the antitumor activity of lenvatinib against human thyroid cancer xenograft models in nude mice. Orally administered lenvatinib showed significant antitumor activity in 5 differentiated thyroid cancer (DTC), 5 anaplastic thyroid cancer (ATC), and 1 medullary thyroid cancer (MTC) xenograft models. Lenvatinib also showed antiangiogenesis activity against 5 DTC and 5 ATC xenografts, while lenvatinib showed in vitro antiproliferative activity against only 2 of 11 thyroid cancer cell lines: that is, RO82-W-1 and TT cells. Western blot analysis showed that cultured RO82-W-1 cells overexpressed FGFR1 and that lenvatinib inhibited the phosphorylation of FGFR1 and its downstream effector FRS2. Lenvatinib also inhibited the phosphorylation of RET with the activated mutation C634W in TT cells. These data demonstrate that lenvatinib provides antitumor activity mainly via angiogenesis inhibition but also inhibits FGFR and RET signaling pathway in preclinical human thyroid cancer models.
Figures

References
-
- Robbins RJ, Wan Q, Grewal RK, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. The Journal of Clinical Endocrinology & Metabolism. 2006;91(2):498–505. - PubMed
-
- Sherman SI. Cytotoxic chemotherapy for differentiated thyroid carcinoma. Clinical Oncology. 2010;22(6):464–468. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

