Interaction analysis through proteomic phage display
- PMID: 25295249
- PMCID: PMC4177731
- DOI: 10.1155/2014/176172
Interaction analysis through proteomic phage display
Abstract
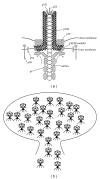
Phage display is a powerful technique for profiling specificities of peptide binding domains. The method is suited for the identification of high-affinity ligands with inhibitor potential when using highly diverse combinatorial peptide phage libraries. Such experiments further provide consensus motifs for genome-wide scanning of ligands of potential biological relevance. A complementary but considerably less explored approach is to display expression products of genomic DNA, cDNA, open reading frames (ORFs), or oligonucleotide libraries designed to encode defined regions of a target proteome on phage particles. One of the main applications of such proteomic libraries has been the elucidation of antibody epitopes. This review is focused on the use of proteomic phage display to uncover protein-protein interactions of potential relevance for cellular function. The method is particularly suited for the discovery of interactions between peptide binding domains and their targets. We discuss the largely unexplored potential of this method in the discovery of domain-motif interactions of potential biological relevance.
Figures

References
-
- Gavin A-C, Maeda K, Kühner S. Recent advances in charting protein-protein interaction: Mass spectrometry-based approaches. Current Opinion in Biotechnology. 2011;22(1):42–49. - PubMed
-
- Dunham WH, Mullin M, Gingras A-C. Affinity-purification coupled to mass spectrometry: basic principles and strategies. Proteomics. 2012;12(10):1576–1590. - PubMed
-
- Barrios-Rodiles M, Brown KR, Ozdamar B, et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science. 2005;307(5715):1621–1625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

