Cytokine-mediated bone destruction in rheumatoid arthritis
- PMID: 25295284
- PMCID: PMC4176903
- DOI: 10.1155/2014/263625
Cytokine-mediated bone destruction in rheumatoid arthritis
Abstract
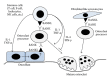
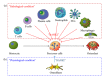
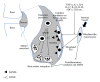
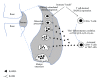
Bone homeostasis, which involves formation and resorption, is an important process for maintaining adequate bone mass in humans. Rheumatoid arthritis (RA) is an autoimmune disease characterized by inflammation and bone loss, leading to joint destruction and deformity, and is a representative disease of disrupted bone homeostasis. The bone loss and joint destruction are mediated by immunological insults by proinflammatory cytokines and various immune cells. The connection between bone and immunity has been intensely studied and comprises the emerging field of osteoimmunology. Osteoimmunology is an interdisciplinary science investigating the interplay between the skeletal and the immune systems. The main contributors in osteoimmunology are the bone effector cells, such as osteoclasts or osteoblasts, and the immune cells, particularly lymphocytes and monocytes. Physiologically, osteoclasts originate from immune cells, and immune cells regulate osteoblasts and vice versa. Pathological conditions such as RA might affect these interactions, thereby altering bone homeostasis, resulting in the unfavorable outcome of bone destruction. In this review, we describe the osteoclastogenic roles of the proinflammatory cytokines and immune cells that are important in the pathophysiology of RA.
Figures

References
-
- Nakashima T, Kobayashi Y, Yamasaki S, et al. Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-κB ligand: modulation of the expression by osteotropic factors and cytokines. Biochemical and Biophysical Research Communications. 2000;275(3):768–775. - PubMed
-
- Kotake S, Nanke Y, Yago T, Kawamoto M, Yamanaka H. Human osteoclastogenic T cells and human osteoclastology. Arthritis and Rheumatism. 2009;60(11):3158–3163. - PubMed
-
- Janssens K, ten Dijke P, Janssens S, van Hul W. Transforming growth factor-β1 to the bone. Endocrine Reviews. 2005;26(6):743–774. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

