A modified γ-retrovirus vector for X-linked severe combined immunodeficiency
- PMID: 25295500
- PMCID: PMC4274995
- DOI: 10.1056/NEJMoa1404588
A modified γ-retrovirus vector for X-linked severe combined immunodeficiency
Abstract
Background: In previous clinical trials involving children with X-linked severe combined immunodeficiency (SCID-X1), a Moloney murine leukemia virus-based γ-retrovirus vector expressing interleukin-2 receptor γ-chain (γc) complementary DNA successfully restored immunity in most patients but resulted in vector-induced leukemia through enhancer-mediated mutagenesis in 25% of patients. We assessed the efficacy and safety of a self-inactivating retrovirus for the treatment of SCID-X1.
Methods: We enrolled nine boys with SCID-X1 in parallel trials in Europe and the United States to evaluate treatment with a self-inactivating (SIN) γ-retrovirus vector containing deletions in viral enhancer sequences expressing γc (SIN-γc).
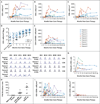
Results: All patients received bone marrow-derived CD34+ cells transduced with the SIN-γc vector, without preparative conditioning. After 12.1 to 38.7 months of follow-up, eight of the nine children were still alive. One patient died from an overwhelming adenoviral infection before reconstitution with genetically modified T cells. Of the remaining eight patients, seven had recovery of peripheral-blood T cells that were functional and led to resolution of infections. The patients remained healthy thereafter. The kinetics of CD3+ T-cell recovery was not significantly different from that observed in previous trials. Assessment of insertion sites in peripheral blood from patients in the current trial as compared with those in previous trials revealed significantly less clustering of insertion sites within LMO2, MECOM, and other lymphoid proto-oncogenes in our patients.
Conclusions: This modified γ-retrovirus vector was found to retain efficacy in the treatment of SCID-X1. The long-term effect of this therapy on leukemogenesis remains unknown. (Funded by the National Institutes of Health and others; ClinicalTrials.gov numbers, NCT01410019, NCT01175239, and NCT01129544.).
Figures

References
-
- Noguchi M, Yi H, Rosenblatt HM, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. - PubMed
-
- Puck JM, Deschênes SM, Porter JC, et al. The interleukin-2 receptor gamma chain maps to Xq13.1 and is mutated in X-linked severe combined immunodeficiency, SCIDX1. Hum Mol Genet. 1993;2:1099–1104. - PubMed
-
- Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366–1369. - PubMed
-
- O’Reilly RJ, Dupont B, Pahwa S, et al. Reconstitution in severe combined immunodeficiency by transplantation of marrow from an unrelated donor. N Engl J Med. 1977;297:1311–1318. - PubMed
-
- Reisner Y, Kapoor N, Kirkpatrick D, et al. Transplantation for severe combined immunodeficiency with HLA-A,B,D,DR incompatible parental marrow cells fractionated by soybean agglutinin and sheep red blood cells. Blood. 1983;61:341–348. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HHSN268201000009C/HL/NHLBI NIH HHS/United States
- U01 AI087628/AI/NIAID NIH HHS/United States
- R01 AI082020/AI/NIAID NIH HHS/United States
- HL 073104/HL/NHLBI NIH HHS/United States
- 269037/ERC_/European Research Council/International
- P30 AI045008/AI/NIAID NIH HHS/United States
- 249816/ERC_/European Research Council/International
- AI 082020/AI/NIAID NIH HHS/United States
- U01 AI087628-05/AI/NIAID NIH HHS/United States
- G0501969/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- P01 HL073104/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
