TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1
- PMID: 25295536
- PMCID: PMC4347231
- DOI: 10.1172/JCI76042
TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1
Abstract
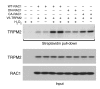
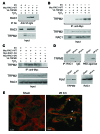
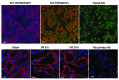
Ischemia is a leading cause of acute kidney injury. Kidney ischemia is associated with loss of cellular ion homeostasis; however, the pathways that underlie ion homeostasis dysfunction are poorly understood. Here, we evaluated the nonselective cation channel transient receptor potential melastatin 2 (TRPM2) in a murine model of kidney ischemia/reperfusion (I/R) injury. TRPM2-deficient mice were resistant to ischemic injury, as reflected by improved kidney function, reduced histologic damage, suppression of proapoptotic pathways, and reduced inflammation. Moreover, pharmacologic TRPM2 inhibition was also protective against I/R injury. TRPM2 was localized mainly in kidney proximal tubule epithelial cells, and studies in chimeric mice indicated that the effects of TRPM2 are due to expression in parenchymal cells rather than hematopoietic cells. TRPM2-deficient mice had less oxidative stress and lower levels of NADPH oxidase activity after ischemia. While RAC1 is a component of the NADPH oxidase complex, its relation to TRPM2 and kidney ischemic injury is unknown. Following kidney ischemia, TRPM2 promoted RAC1 activation, with active RAC1 physically interacting with TRPM2 and increasing TRPM2 expression at the cell membrane. Finally, inhibition of RAC1 reduced oxidant stress and ischemic injury in vivo. These results demonstrate that TRPM2-dependent RAC1 activation increases oxidant stress and suggest that therapeutic approaches targeting TRPM2 and/or RAC1 may be effective in reducing ischemic kidney injury.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

