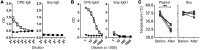Skin exposure promotes a Th2-dependent sensitization to peanut allergens
- PMID: 25295541
- PMCID: PMC4347216
- DOI: 10.1172/JCI75660
Skin exposure promotes a Th2-dependent sensitization to peanut allergens
Abstract
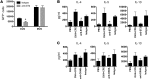
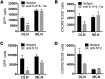
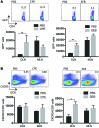
Sensitization to foods often occurs in infancy, without a known prior oral exposure, suggesting that alternative exposure routes contribute to food allergy. Here, we tested the hypothesis that peanut proteins activate innate immune pathways in the skin that promote sensitization. We exposed mice to peanut protein extract on undamaged areas of skin and observed that repeated topical exposure to peanut allergens led to sensitization and anaphylaxis upon rechallenge. In mice, this epicutaneous peanut exposure induced sensitization to the peanut components Ara h 1 and Ara h 2, which is also observed in human peanut allergy. Both crude peanut extract and Ara h 2 alone served as adjuvants, as both induced a bystander sensitization that was similar to that induced by the atopic dermatitis-associated staphylococcal enterotoxin B. In cultured human keratinocytes and in murine skin, peanut extract directly induced cytokine expression. Moreover, topical peanut extract application induced an alteration dependent on the IL-33 receptor ST2 in skin-draining DCs, resulting in Th2 cytokine production from T cells. Together, our data support the hypothesis that peanuts are allergenic due to inherent adjuvant activity and suggest that skin exposure to food allergens contributes to sensitization to foods in early life.
Figures