Endoplasmic reticulum stress as a novel mechanism in amiodarone-induced destructive thyroiditis
- PMID: 25295624
- PMCID: PMC4283007
- DOI: 10.1210/jc.2014-2745
Endoplasmic reticulum stress as a novel mechanism in amiodarone-induced destructive thyroiditis
Abstract
Context: Amiodarone (AMIO) is one of the most effective antiarrhythmic drugs available; however, its use is limited by a serious side effect profile, including thyroiditis. The mechanisms underlying AMIO thyroid toxicity have been elusive; thus, identification of novel approaches in order to prevent thyroiditis is essential in patients treated with AMIO.
Objective: Our aim was to evaluate whether AMIO treatment could induce endoplasmic reticulum (ER) stress in human thyroid cells and the possible implications of this effect in AMIO-induced destructive thyroiditis.
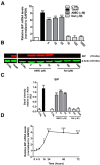
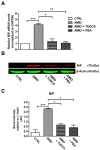
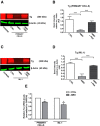
Results: Here we report that AMIO, but not iodine, significantly induced the expression of ER stress markers including Ig heavy chain-binding protein (BiP), phosphoeukaryotic translation initiation factor 2α (eIF2α), CCAAT/enhancer-binding protein homologous protein (CHOP) and spliced X-box binding protein-1 (XBP-1) in human thyroid ML-1 cells and human primary thyrocytes. In both experimental systems AMIO down-regulated thyroglobulin (Tg) protein but had little effect on Tg mRNA levels, suggesting a mechanism involving Tg protein degradation. Indeed, pretreatment with the specific proteasome inhibitor MG132 reversed AMIO-induced down-regulation of Tg protein levels, confirming a proteasome-dependent degradation of Tg protein. Corroborating our findings, pretreatment of ML-1 cells and human primary thyrocytes with the chemical chaperone 4-phenylbutyric acid completely prevented the effect of AMIO on both ER stress induction and Tg down-regulation.
Conclusions: We identified ER stress as a novel mechanism contributing to AMIO-induced destructive thyroiditis. Our data establish that AMIO-induced ER stress impairs Tg expression via proteasome activation, providing a valuable therapeutic avenue for the treatment of AMIO-induced destructive thyroiditis.
Figures



Similar articles
-
Punicalagin exerts protective effect against high glucose-induced cellular stress and neural tube defects.Biochem Biophys Res Commun. 2015 Nov 13;467(2):179-84. doi: 10.1016/j.bbrc.2015.10.024. Epub 2015 Oct 13. Biochem Biophys Res Commun. 2015. Retraction in: Biochem Biophys Res Commun. 2025 Sep 11:152601. doi: 10.1016/j.bbrc.2025.152601. PMID: 26453010 Free PMC article. Retracted.
-
Selective, potent blockade of the IRE1 and ATF6 pathways by 4-phenylbutyric acid analogues.Br J Pharmacol. 2013 Oct;170(4):822-34. doi: 10.1111/bph.12306. Br J Pharmacol. 2013. PMID: 23869584 Free PMC article.
-
Activation of volume-sensitive outwardly rectifying chloride channel by ROS contributes to ER stress and cardiac contractile dysfunction: involvement of CHOP through Wnt.Cell Death Dis. 2014 Nov 20;5(11):e1528. doi: 10.1038/cddis.2014.479. Cell Death Dis. 2014. PMID: 25412307 Free PMC article.
-
Unfolded protein response is involved in the pathology of human congenital hypothyroid goiter and rat non-goitrous congenital hypothyroidism.J Mol Endocrinol. 2004 Jun;32(3):903-20. doi: 10.1677/jme.0.0320903. J Mol Endocrinol. 2004. PMID: 15171721
-
A sigma-1 receptor antagonist (NE-100) prevents tunicamycin-induced cell death via GRP78 induction in hippocampal cells.Biochem Biophys Res Commun. 2013 May 17;434(4):904-9. doi: 10.1016/j.bbrc.2013.04.055. Epub 2013 Apr 22. Biochem Biophys Res Commun. 2013. PMID: 23618865
Cited by
-
Interferon alpha: The key trigger of type 1 diabetes.J Autoimmun. 2018 Nov;94:7-15. doi: 10.1016/j.jaut.2018.08.003. Epub 2018 Aug 14. J Autoimmun. 2018. PMID: 30115527 Free PMC article. Review.
-
Hepatitis C Virus Infection of Human Thyrocytes: Metabolic, Hormonal, and Immunological Implications.J Clin Endocrinol Metab. 2020 Apr 1;105(4):1157-68. doi: 10.1210/clinem/dgz241. J Clin Endocrinol Metab. 2020. PMID: 31784757 Free PMC article.
-
Abnormal Glucose Metabolism and Insulin Resistance Are Induced via the IRE1α/XBP-1 Pathway in Subclinical Hypothyroidism.Front Endocrinol (Lausanne). 2019 May 17;10:303. doi: 10.3389/fendo.2019.00303. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31156553 Free PMC article.
-
Evaluation of autophagy inducers in epithelial cells carrying the ΔF508 mutation of the cystic fibrosis transmembrane conductance regulator CFTR.Cell Death Dis. 2018 Feb 7;9(2):191. doi: 10.1038/s41419-017-0235-9. Cell Death Dis. 2018. PMID: 29415993 Free PMC article.
-
Sirolimus induces depletion of intracellular calcium stores and mitochondrial dysfunction in pancreatic beta cells.Sci Rep. 2017 Nov 20;7(1):15823. doi: 10.1038/s41598-017-15283-y. Sci Rep. 2017. PMID: 29158477 Free PMC article.
References
-
- Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–e198. - PubMed
-
- Vassallo P, Trohman RG. Prescribing amiodarone: an evidence-based review of clinical indications. JAMA. 2007;298:1312–1322. - PubMed
-
- Han TS, Williams GR, Vanderpump MP. Benzofuran derivatives and the thyroid. Clin Endocrinol (Oxf). 2009;70:2–13. - PubMed
-
- Ahmed S, Van Gelder IC, Wiesfeld AC, Van Veldhuisen DJ, Links TP. Determinants and outcome of amiodarone-associated thyroid dysfunction. Clin Endocrinol (Oxf). 2011;75:388–394. - PubMed
-
- Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: meta-analysis of individual data from 6500 patients in randomised trials. Amiodarone Trials Meta-Analysis Investigators. Lancet. 1997;350:1417–1424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

