Novel nano-sized MR contrast agent mediates strong tumor contrast enhancement in an oncogene-driven breast cancer model
- PMID: 25296030
- PMCID: PMC4189783
- DOI: 10.1371/journal.pone.0107762
Novel nano-sized MR contrast agent mediates strong tumor contrast enhancement in an oncogene-driven breast cancer model
Abstract
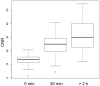
The current study was carried out to test the potential of a new nanomaterial (Spago Pix) as a macromolecular magnetic MR contrast agent for tumor detection and to verify the presence of nanomaterial in tumor tissue. Spago Pix, synthesized by Spago Nanomedical AB, is a nanomaterial with a globular shape, an average hydrodynamic diameter of 5 nm, and a relaxivity (r1) of approximately 30 (mM Mn)-1 s-1 (60 MHz). The material consists of an organophosphosilane hydrogel with strongly chelated manganese (II) ions and a covalently attached PEG surface layer. In vivo MRI of the MMTV-PyMT breast cancer model was performed on a 3 T clinical scanner. Tissues were thereafter analyzed for manganese and silicon content using inductively coupled plasma-atomic emission spectroscopy (ICP-AES). The presence of nanomaterial in tumor and muscle tissue was assessed using an anti-PEG monoclonal antibody. MR imaging of tumor-bearing mice (n = 7) showed a contrast enhancement factor of 1.8 (tumor versus muscle) at 30 minutes post-administration. Contrast was retained and further increased 2-4 hours after administration. ICP-AES and immunohistochemistry confirmed selective accumulation of nanomaterial in tumor tissue. A blood pharmacokinetics analysis showed that the concentration of Spago Pix gradually decreased over the first hour, which was in good agreement with the time frame in which the accumulation in tumor occurred. In summary, we demonstrate that Spago Pix selectively enhances MR tumor contrast in a clinically relevant animal model. Based on the generally higher vascular leakiness in malignant compared to benign tissue lesions, Spago Pix has the potential to significantly improve cancer diagnosis and characterization by MRI.
Conflict of interest statement
Figures







References
-
- Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, et al. (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging JMRI 10: 223–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

