Neurophysiological correlates of sevoflurane-induced unconsciousness
- PMID: 25296108
- PMCID: PMC4301983
- DOI: 10.1097/ALN.0000000000000482
Neurophysiological correlates of sevoflurane-induced unconsciousness
Abstract
Background: Recent studies of anesthetic-induced unconsciousness in humans have focused predominantly on the intravenous drug propofol and have identified anterior dominance of alpha rhythms and frontal phase-amplitude coupling patterns as neurophysiological markers. However, it is unclear whether the correlates of propofol-induced unconsciousness are generalizable to inhaled anesthetics, which have distinct molecular targets and which are used more commonly in clinical practice.
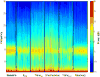
Methods: The authors recorded 64-channel electroencephalograms in healthy human participants during consciousness, sevoflurane-induced unconsciousness, and recovery (n = 10; n = 7 suitable for analysis). Spectrograms and scalp distributions of low-frequency (1 Hz) and alpha (10 Hz) power were analyzed, and phase-amplitude modulation between these two frequencies was calculated in frontal and parietal regions. Phase lag index was used to assess phase relationships across the cortex.
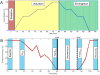
Results: At concentrations sufficient for unconsciousness, sevoflurane did not result in a consistent anteriorization of alpha power; the relationship between low-frequency phase and alpha amplitude in the frontal cortex did not undergo characteristic transitions. By contrast, there was significant cross-frequency coupling in the parietal region during consciousness that was not observed after loss of consciousness. Furthermore, a reversible disruption of anterior-posterior phase relationships in the alpha bandwidth was identified as a correlate of sevoflurane-induced unconsciousness.
Conclusion: In humans, sevoflurane-induced unconsciousness is not correlated with anteriorization of alpha and related cross-frequency patterns, but rather by a disruption of phase-amplitude coupling in the parietal region and phase-phase relationships across the cortex.
Conflict of interest statement
Figures



References
-
- Purdon PL, Pierce ET, Mukamel EA, Prerau MJ, Walsh JL, Wong KFK, Salazar-Gomez AF, Harrell PG, Sampson AL, Cimenser A, Ching S, Kopell NJ, Tavares-Stoeckel C, Habeeb K, Merhar R, Brown EN. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci. 2013;110:E1142–51. - PMC - PubMed
-
- Mhuircheartaigh RN, Warnaby C, Rogers R, Jbabdi S, Tracey I. Slow-wave activity saturation and thalamocortical isolation during propofol anesthesia in humans. Sci Transl Med. 2013;5:208ra148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

