Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network
- PMID: 25296246
- PMCID: PMC4214411
- DOI: 10.1038/ncomms6124
Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network
Abstract
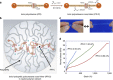
Stimuli-sensitive hydrogels changing their volumes and shapes in response to various stimulations have potential applications in multiple fields. However, these hydrogels have not yet been commercialized due to some problems that need to be overcome. One of the most significant problems is that conventional stimuli-sensitive hydrogels are usually brittle. Here we prepare extremely stretchable thermosensitive hydrogels with good toughness by using polyrotaxane derivatives composed of α-cyclodextrin and polyethylene glycol as cross-linkers and introducing ionic groups into the polymer network. The ionic groups help the polyrotaxane cross-linkers to become well extended in the polymer network. The resulting hydrogels are surprisingly stretchable and tough because the cross-linked α-cyclodextrin molecules can move along the polyethylene glycol chains. In addition, the polyrotaxane cross-linkers can be used with a variety of vinyl monomers; the mechanical properties of the wide variety of polymer gels can be improved by using these cross-linkers.
Figures




References
-
- Osada Y. et al. Gels Handbook: The Fundamentals Elsevier Inc. (2001).
-
- Tanaka T. Gels. Sci. Am. 244, 124–136 (1981). - PubMed
-
- Okano T., Bae Y. H., Jacobs H. & Kim S. W. Thermally on off switching polymers for drug permeation and release. J. Control. Rel. 11, 255–265 (1990).
-
- Calvert P. Hydrogels for soft machines. Adv. Mater. 21, 743–756 (2009).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

