The mechanism of phagocytosis: two stages of engulfment
- PMID: 25296306
- PMCID: PMC4190621
- DOI: 10.1016/j.bpj.2014.07.070
The mechanism of phagocytosis: two stages of engulfment
Abstract
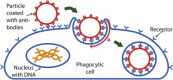
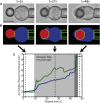
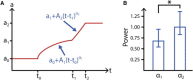
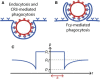
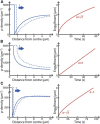
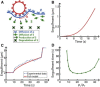
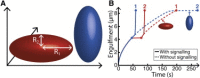
Despite being of vital importance to the immune system, the mechanism by which cells engulf relatively large solid particles during phagocytosis is still poorly understood. From movies of neutrophil phagocytosis of polystyrene beads, we measure the fractional engulfment as a function of time and demonstrate that phagocytosis occurs in two distinct stages. During the first stage, engulfment is relatively slow and progressively slows down as phagocytosis proceeds. However, at approximately half-engulfment, the rate of engulfment increases dramatically, with complete engulfment attained soon afterwards. By studying simple mathematical models of phagocytosis, we suggest that the first stage is due to a passive mechanism, determined by receptor diffusion and capture, whereas the second stage is more actively controlled, perhaps with receptors being driven toward the site of engulfment. We then consider a more advanced model that includes signaling and captures both stages of engulfment. This model predicts that there is an optimum ligand density for quick engulfment. Further, we show how this model explains why nonspherical particles engulf quickest when presented tip-first. Our findings suggest that active regulation may be a later evolutionary innovation, allowing fast and robust engulfment even for large particles.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

