Potentiation of neurotoxicity in double-mutant mice with Pink1 ablation and A53T-SNCA overexpression
- PMID: 25296918
- PMCID: PMC4986551
- DOI: 10.1093/hmg/ddu520
Potentiation of neurotoxicity in double-mutant mice with Pink1 ablation and A53T-SNCA overexpression
Abstract
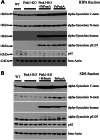
The common age-related neurodegeneration of Parkinson's disease can result from dominant causes like increased dosage of vesicle-associated alpha-synuclein (SNCA) or recessive causes like deficiency of mitophagy factor PINK1. Interactions between these triggers and their convergence onto shared pathways are crucial, but currently conflicting evidence exists. Here, we crossed previously characterized mice with A53T-SNCA overexpression and with Pink1 deletion to generate double mutants (DMs). We studied their lifespan and behavior, histological and molecular anomalies at late and early ages. DM animals showed potentiated phenotypes in comparison with both single mutants (SMs), with reduced survival and strongly reduced spontaneous movements from the age of 3 months onwards. In contrast to SMs, a quarter of DM animals manifested progressive paralysis at ages >1 year and exhibited protein aggregates immunopositive for pSer129-SNCA, p62 and ubiquitin in spinal cord and basal brain. Brain proteome quantifications of ubiquitination sites documented altered degradation of SNCA and the DNA-damage marker H2AX at the age of 18 months. Global brain transcriptome profiles and qPCR validation experiments identified many consistent transcriptional dysregulations already at the age of 6 weeks, which were absent from SMs. The observed downregulations for Dapk1, Dcaf17, Rab42 and the novel SNCA-marker Lect1 as well as the upregulations for Dctn5, Mrpl9, Tmem181a, Xaf1 and H2afx reflect changes in ubiquitination, mitochondrial/synaptic/microtubular/cell adhesion dynamics and DNA damage. Thus, our study confirmed that SNCA-triggered neurotoxicity is exacerbated by the absence of PINK1 and identified a novel molecular signature that is detectable early in the course of this double pathology.
© The Author 2014. Published by Oxford University Press.
Figures

Similar articles
-
Progression of pathology in PINK1-deficient mouse brain from splicing via ubiquitination, ER stress, and mitophagy changes to neuroinflammation.J Neuroinflammation. 2017 Aug 2;14(1):154. doi: 10.1186/s12974-017-0928-0. J Neuroinflammation. 2017. PMID: 28768533 Free PMC article.
-
SerThr-PhosphoProteome of Brain from Aged PINK1-KO+A53T-SNCA Mice Reveals pT1928-MAP1B and pS3781-ANK2 Deficits, as Hub between Autophagy and Synapse Changes.Int J Mol Sci. 2019 Jul 4;20(13):3284. doi: 10.3390/ijms20133284. Int J Mol Sci. 2019. PMID: 31277379 Free PMC article.
-
Prodromal sensory neuropathy in Pink1-/- SNCAA53T double mutant Parkinson mice.Neuropathol Appl Neurobiol. 2021 Dec;47(7):1060-1079. doi: 10.1111/nan.12734. Epub 2021 May 28. Neuropathol Appl Neurobiol. 2021. PMID: 33974284
-
A53T in a parkinsonian family: a clinical update of the SNCA phenotypes.J Neural Transm (Vienna). 2016 Nov;123(11):1301-1307. doi: 10.1007/s00702-016-1578-6. Epub 2016 Jun 1. J Neural Transm (Vienna). 2016. PMID: 27250986 Review.
-
Molecular insights into Parkinson's disease.Prog Mol Biol Transl Sci. 2012;107:125-88. doi: 10.1016/B978-0-12-385883-2.00011-4. Prog Mol Biol Transl Sci. 2012. PMID: 22482450 Review.
Cited by
-
Impaired Photic Entrainment of Spontaneous Locomotor Activity in Mice Overexpressing Human Mutant α-Synuclein.Int J Mol Sci. 2018 Jun 3;19(6):1651. doi: 10.3390/ijms19061651. Int J Mol Sci. 2018. PMID: 29865270 Free PMC article.
-
Lack of PINK1 alters glia innate immune responses and enhances inflammation-induced, nitric oxide-mediated neuron death.Sci Rep. 2018 Jan 10;8(1):383. doi: 10.1038/s41598-017-18786-w. Sci Rep. 2018. PMID: 29321620 Free PMC article.
-
Progression of pathology in PINK1-deficient mouse brain from splicing via ubiquitination, ER stress, and mitophagy changes to neuroinflammation.J Neuroinflammation. 2017 Aug 2;14(1):154. doi: 10.1186/s12974-017-0928-0. J Neuroinflammation. 2017. PMID: 28768533 Free PMC article.
-
Liquid-liquid phase separation and conformational strains of α-Synuclein: implications for Parkinson's disease pathogenesis.Front Mol Neurosci. 2024 Oct 23;17:1494218. doi: 10.3389/fnmol.2024.1494218. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39507104 Free PMC article. Review.
-
PTEN-Induced Putative Kinase 1 Dysfunction Accelerates Synucleinopathy.J Parkinsons Dis. 2022;12(4):1201-1217. doi: 10.3233/JPD-213065. J Parkinsons Dis. 2022. PMID: 35253778 Free PMC article.
References
-
- Corti O., Lesage S., Brice A. What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiol. Rev. 2011;91:1161–1218. - PubMed
-
- Trinh J., Farrer M. Advances in the genetics of Parkinson disease. Nat. Rev. Neurol. 2013;9:445–454. - PubMed
-
- Hardy J., Bogdanovic N., Winblad B., Portelius E., Andreasen N., Cedazo-Minguez A., Zetterberg H. Pathways to Alzheimer's disease. J. Intern. Med. 2014;275:296–303. - PubMed
-
- Polymeropoulos M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

