Fluorescent labeling for clonal selection of Marc 145 cells secreting high levels of recombinant protein PBD-1
- PMID: 25297006
- PMCID: PMC4754255
- DOI: 10.1007/s10616-014-9769-1
Fluorescent labeling for clonal selection of Marc 145 cells secreting high levels of recombinant protein PBD-1
Abstract
Despite the powerful impact gene expression markers like the green fluorescent protein (GFP) or enhanced GFP (EGFP) exert on linking the expression of recombinant protein for selection of high producers in recent years, there is still a strong incentive to develop more economical and efficient methods for isolating mammalian cell clones secreting high levels of recombinant proteins. Here we present a new method based on the co-expression of EGFP that allows clonal selection in standard 96-well cell culture plates. The genes encoding the EGFP protein and the related protein are linked by an internal ribosome entry site and thus are transcribed into the same mRNA in an independent translation process. Since both proteins arise from a common mRNA, the EGFP expression level correlates with the expression level of the therapeutic protein in each clone. By expressing recombinant porcine β-defensin 1 in Marc 145 cells, we demonstrate the robustness and performance of this technique. The method can be served as an alternative to identify high-producer clones with various cell sorting methods.
Keywords: High producer; Mammalian cell culture; Porcine β-defensin l; Recombinant protein.
Figures
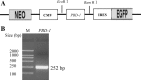

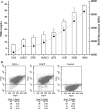
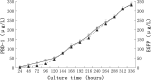

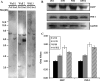
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

