Motor cortex is functionally organized as a set of spatially distinct representations for complex movements
- PMID: 25297087
- PMCID: PMC6608383
- DOI: 10.1523/JNEUROSCI.2500-14.2014
Motor cortex is functionally organized as a set of spatially distinct representations for complex movements
Abstract
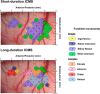
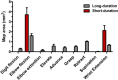
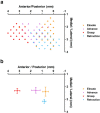
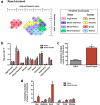
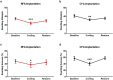
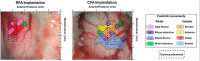
There is a long-standing debate regarding the functional organization of motor cortex. Intracortical microstimulation (ICMS) studies have provided two contrasting views depending on the duration of stimulation. In the rat, short-duration ICMS reveals two spatially distributed forelimb movement representations, the rostral forelimb area (RFA) and caudal forelimb area (CFA), eliciting identical movements. In contrast, long-duration ICMS reveals spatially distributed, complex, multijoint movement areas, with grasping found exclusively in the rostral area and reach-shaping movements of the arm located in the caudal area. To provide corroboration for which interpretation is correct, we selectively inactivated the RFA/grasp area during the performance of skilled forelimb behaviors using a reversible cortical cooling deactivation technique. A significant impairment of grasping in the single-pellet retrieval task and manipulations of pasta was observed during cooling deactivation of the RFA/grasp area, but not the CFA/arm area. Our results indicate a movement-based, rather than a muscle-based, functional organization of motor cortex, and provide evidence for a conserved homology of independent grasp and reach circuitry shared between primates and rats.
Keywords: behavior; intracortical microstimulation; rat; reversible lesion.
Copyright © 2014 the authors 0270-6474/14/3413574-12$15.00/0.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
