Alzheimer's disease-like pathology induced by amyloid-β oligomers in nonhuman primates
- PMID: 25297091
- PMCID: PMC6608380
- DOI: 10.1523/JNEUROSCI.1353-14.2014
Alzheimer's disease-like pathology induced by amyloid-β oligomers in nonhuman primates
Erratum in
-
Erratum: Forny-Germano et al., "Alzheimer's Disease-Like Pathology Induced by Amyloid-β Oligomers in Nonhuman Primates".J Neurosci. 2020 Oct 14;40(42):8204. doi: 10.1523/JNEUROSCI.1827-20.2020. Epub 2020 Oct 7. J Neurosci. 2020. PMID: 33028640 Free PMC article. No abstract available.
Abstract
Alzheimer's disease (AD) is a devastating neurodegenerative disorder and a major medical problem. Here, we have investigated the impact of amyloid-β (Aβ) oligomers, AD-related neurotoxins, in the brains of rats and adult nonhuman primates (cynomolgus macaques). Soluble Aβ oligomers are known to accumulate in the brains of AD patients and correlate with disease-associated cognitive dysfunction. When injected into the lateral ventricle of rats and macaques, Aβ oligomers diffused into the brain and accumulated in several regions associated with memory and cognitive functions. Cardinal features of AD pathology, including synapse loss, tau hyperphosphorylation, astrocyte and microglial activation, were observed in regions of the macaque brain where Aβ oligomers were abundantly detected. Most importantly, oligomer injections induced AD-type neurofibrillary tangle formation in the macaque brain. These outcomes were specifically associated with Aβ oligomers, as fibrillar amyloid deposits were not detected in oligomer-injected brains. Human and macaque brains share significant similarities in terms of overall architecture and functional networks. Thus, generation of a macaque model of AD that links Aβ oligomers to tau and synaptic pathology has the potential to greatly advance our understanding of mechanisms centrally implicated in AD pathogenesis. Furthermore, development of disease-modifying therapeutics for AD has been hampered by the difficulty in translating therapies that work in rodents to humans. This new approach may be a highly relevant nonhuman primate model for testing therapeutic interventions for AD.
Keywords: Alzheimer's disease; amyloid-β oligomers; nonhuman primate; synapse loss; tau pathology.
Copyright © 2014 the authors 0270-6474/14/3413629-15$15.00/0.
Figures


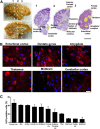
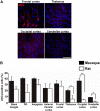
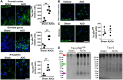




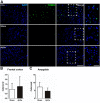

References
-
- Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease- associated Abeta oligomers. J Clin Invest. 2012;122:1339–1353. doi: 10.1172/JCI57256. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
